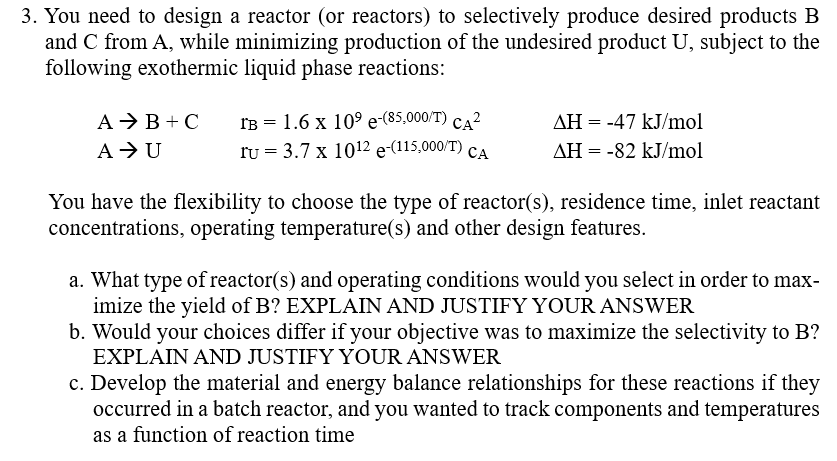
Question: You need to design a reactor ( or reactors ) to selectively produce desired products B and C from A , while minimizing production of
You need to design a reactor or reactors to selectively produce desired products
and from while minimizing production of the undesired product subject to the
following exothermic liquid phase reactions:
You have the flexibility to choose the type of reactors residence time, inlet reactant
concentrations, operating temperatures and other design features.
a What type of reactors and operating conditions would you select in order to max
imize the yield of B EXPLAIN AND JUSTIFY YOUR ANSWER
b Would your choices differ if your objective was to maximize the selectivity to B
EXPLAIN AND JUSTIFY YOUR ANSWER
c Develop the material and energy balance relationships for these reactions if they
occurred in a batch reactor, and you wanted to track components and temperatures
as a function of reaction time
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


