Question: Using the thermal diffusion apparatus of Example 3.22 with two bulbs at 0 C and 123 C, respectively, estimate the mole-fraction difference in
Using the thermal diffusion apparatus of Example 3.22 with two bulbs at 0οC and 123οC, respectively, estimate the mole-fraction difference in H2 at steady state from a mixture initially consisting of mole fractions 0.1 and 0.9 for D2 and H2, respectively.
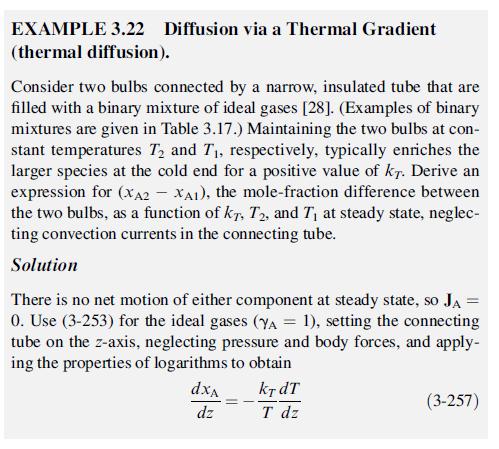
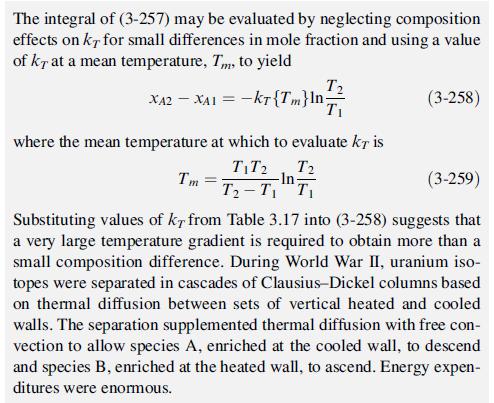
EXAMPLE 3.22 Diffusion via a Thermal Gradient (thermal diffusion). Consider two bulbs connected by a narrow, insulated tube that are filled with a binary mixture of ideal gases [28]. (Examples of binary mixtures are given in Table 3.17.) Maintaining the two bulbs at con- stant temperatures 7 and T, respectively, typically enriches the larger species at the cold end for a positive value of kr. Derive an expression for (XA2 - XA1), the mole-fraction difference between the two bulbs, as a function of kr, T2, and T at steady state, neglec- ting convection currents in the connecting tube. Solution = There is no net motion of either component at steady state, so JA 0. Use (3-253) for the ideal gases (YA = 1), setting the connecting tube on the z-axis, neglecting pressure and body forces, and apply- ing the properties of logarithms to obtain dxA dz kt dT T dz (3-257)
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
To estimate the molefraction difference in H2 at steady state in the thermal diffusion apparatus des... View full answer

Get step-by-step solutions from verified subject matter experts


