The black modification of SnO is isostructural with PbO litharge of the previous problem. (a) The bond-valence
Question:
The black modification of SnO is isostructural with PbO litharge of the previous problem.
(a) The bond-valence parameters for Sn2+–O are R0 = 1.98 Å and B = 0.37 Å. Use Equation (5.18) (dij = ¼R0ij - Blnvij) to predict the Sn–O bond length in the hypothetical CsCl-type SnO. Use this distance to calculate the unit-cell edge and volume.
(b) The crystal structure of SnO has space group P4mm and Z = 2, with a = 3.803 Å, c = 4.838 Å. Calculate the unit-cell volume per formula unit and compare with your prediction of the volume for “cubic” SnO with the CsCl structure. What percent expansion is needed to make room for the stereo chemically active electron lone pair?
Equation (5.18)
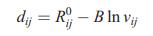
Step by Step Answer:

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt





