Question: The -cristobalite polymorph of SiO 2 has a tetragonal cell with a = 4.971 , b = 6.922 , with Si on 2a and O
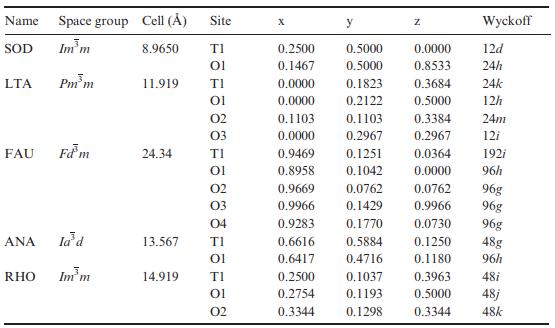
The α-cristobalite polymorph of SiO2 has a tetragonal cell with a = 4.971 Å, b = 6.922 Å, with Si on 2a and O on 4f Wyckoff sites. The Si–O bond length is 1.60 Å and the average O–O distance within tetrahedra is 2.62 Å. The high-pressure stishovite polymorph of SiO2 has the rutile structure (Figure 1.45) with a tetragonal cell of dimensions a = 4.177 Å and c = 2.665 Å, and occupied Wyckoff sites of 2a (Si) and 4f (O). Data for a zeolite A polymorph of SiO2 (LTA) are given in the table accompanying Problem 14.4.
(a) Calculate the theoretical density of each polymorph.
(b) Assuming that Si and O can be treated as hard spheres and that O atoms are in contact in cristobalite, estimate hard-sphere radii for Si and O.
(c) Estimate the volume percent filled by atoms in each polymorph. Comment on your answers.
Figure 1.45
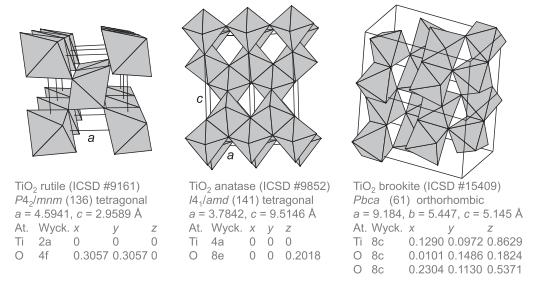
Problem 14.4
Calculate the number of tetrahedral atoms per nm3 for each zeolite framework type listed in the table below.
X TiO rutile (ICSD # 9161) P4 /mnm (136) tetragonal a = 4.5941, c= 2.9589 A At. Wyck. x Ti 2a 0 y 0 z 0 O4f 0.3057 0.3057 0 TiO anatase (ICSD # 9852) 14./amd (141) tetragonal a= 3.7842, c= 9.5146 A At. Wyck. x y z Ti 4a 000 O 8e 0 0 0.2018 TiO brookite (ICSD #15409) Pbca (61) orthorhombic a = 9.184, b= 5.447, c = 5.145 A At. Wyck. x y Ti 8c z 0 8c O 8c 0.1290 0.0972 0.8629 0.0101 0.1486 0.1824 0.2304 0.1130 0.5371
Step by Step Solution
3.43 Rating (150 Votes )
There are 3 Steps involved in it
This Problem gives some feel for the high accessible volume of zeolite struct... View full answer

Get step-by-step solutions from verified subject matter experts


