Question: Methanol is produced using syngas, a mixture (CO12 H2) containing CO2 as an inert impurity. Originally there were 0.25 mol per 100 mol of syngas.
Methanol is produced using syngas, a mixture (CO12 H2) containing CO2 as an inert impurity. Originally there were 0.25 mol per 100 mol of syngas. The reactor operates at 25 bar and 232C. Assume that 90% of the methanol can be recovered condensing it. No water is produced since Co-based catalysts are used. The reactor can handle up to 5 moles of CO2 per 100 moles of syngas. Fig. P5.15 shows the scheme of the synthesis loop. Determine:
1. The reactor conversion assuming that the equilibrium constant of the process taking place in the reactor can be computed using the following equation:
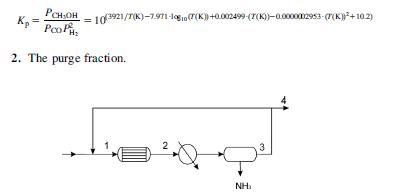
KpPoots 103921/7(K)-7.971-100(7(K))+0.002499-(7(K))-0.0000002953-((K)+10.2) 2. The purge fraction. 2 NH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


