Starting from the MaxwellBoltzmann distribution, eq. (18.10) for the probability of finding a particle with energy E
Question:
Starting from the Maxwell–Boltzmann distribution, eq. (18.10)
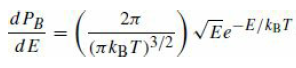
for the probability of finding a particle with energy E in a gas with temperature T, show that the most probable speed for a particle is v̅ = √2kBT/m, but that the average speed of a particle is 〈v〉 = √8kBT/πm. Verify that the most probable speed for a neutron at T = 20◦C is ∼2200m/s.
Transcribed Image Text:
dPB VEe-E/kBT (7 kB T)3/2 dE
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
First we change variable from E to v using E mv 2 2 and dE mv dv To ...View the full answer

Answered By

AJIN kuriakose
I have completed B.Tech in Electrical Engineering & Masters in Power & Control From one of the best universities in India. I got the 99.05 percentile in the Gate Electrical Engineering Exam. I can Help students solving assignments in Electrical subjects like Power Electronics, Control system, Analog, Network Theory & Engineering Mathematics. Clear your fundamentals and develop problem-solving skills and analytical skills to crack the exam.
Get guidance and the opportunity to learn from experienced...
I can provide tuition for Electrical engineering subjects (Power Electronics, Digital electronics, Network Theory, Control System & Engineering Mathematics). The toughest subject of Electrical engineering can be made simple in online classes...
I can also solve it.
1 .I can help you with your assignments or exams or quiz or tutoring.
2. Very strict to the deadlines.
Message me for any help in assignments, live sessions. I am here to help students for all assignments, tests and exams and I will make sure you always get _95% In your subject.
Contact me in solution inn for any help in your semester, projects and for many more things . Also feel free to contact me through solution inn and for any advise related to tutoring and how it works here.thank you.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Show that the probability of finding a particle with energy greater than 0.2 MeV in a gas at temperature 15 10 6 K is 10 69 .
-
What is the probability of finding a particle in a box of length L in the region between x = L/4 and x = 3L/4 when the particle is in (a) The ground level and (b) The first excited level? (c) Are...
-
Some consequences of the Maxwell-Boltzmann distribution, in the simplified kinetic theory in S1.4, several statements concerning the equilibrium behavior of a gas were made without proof. In this...
-
Simplify the expression 2x + (x + 1) into a single x + 1 fraction. The numerator of your answer is: The denominator of your answer is:
-
The space shuttle travels at a speed of about 7.6 103 m/s. The blink of an astronaut's eye lasts about 110 ms. How many football fields (length = 91.4 m) does the shuttle cover in the blink of an...
-
Compute the selling price of 10%, 15-year bonds with a par value of $240,000 and semiannual interest payments. The annual market rate for these bonds is 8%. Use present value tables B.1 and B.3 in...
-
You want to retire in 30 years. You are starting to invest in a growth income fund that promises an ambitious rate of $15 \%$. You can put in $\$ 200$ per month. How much will you have in 30 years?
-
A firm is concerned about the condition of some of its plant machinery. Bill James, a newly hired engineer, was assigned the task of reviewing the situation and determining what alternatives are...
-
On April 1, 2020, Gusto Corp. purchases a call option for $500, giving Gusto the right to purchase 1,000 shares of Delta Inc. for $30 each until December 1, 2020. Delta Stock Inc. are currently...
-
1. What business research problem does Royal Barton face? What are his information needs? Outline some survey research objectives for a research project on the Royal Bee system. 2. What type of...
-
Tritium is just barely unstable. How much more tightly would tritium have to be bound in order for it to bestable? Write the nuclear reaction describing tt fusion.How much energy is emitted in tt...
-
Show from eq. (19.9) that in an infinite, homogeneous graphite-moderated reactor, the resonance escape probability is given by p(y) = exp(?17.28 (1/(9.3 + 4.9y)) 0.514 ), where y is the ratio of...
-
I recently discovered a great new tool called Google Finance. Using Google Finance with the Google spreadsheet makes it possible to look up all kinds of data on mutual funds. So, of course I...
-
When companies have excess cash beyond their needs, they can return this money to shareholders in the form of a dividend or through a stock repurchase. Differentiate the effects of repurchases and...
-
Marcus Holloway has $7500 that he will use as a down payment on a car. Assuming that he can afford a payment of $625 per month, how much can Marcus spend on a car (that is, what is the total cost of...
-
Phone Corporation owns a 90% interest in Style Company, acquired several years ago at a cost equal to book value and fair value. Style sells merchandise to Phone for the first time in 2023, and some...
-
Fred Amigo, a resident taxpayer aged 58, is the recipient of a superannuation pension, which commenced on 1 July 2018. Theannualpension amountis$4O,OOO.PAYGtaxof$510waswithheldfrom the pension. ...
-
Critically analyze the AI's response. Discuss how you would revise and improve upon the information provided by the AI , incorporating your knowledge and insights as a financial expert.
-
Which is the accounting statement that reports in detail, and for a very specific time period, a businesss revenue, the expenses required to generate its revenues, and the businesss profits or losses...
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
A chirp signal, in which the angular frequency rises uniformly from Ï 0 ÎÏ/2 to Ï 0 + ÎÏ/2 over a time T, can be written as exp(iÏ(t)), where the phase Ï(t)...
-
For microwave radiation propagating vertically through the Earths atmosphere, the delay to a pulse as a result of the dry component of the atmosphere, expressed as an equivalent distance, is 2.33...
-
Prove Equation (8.16). Equation 8.16 dP, P,(t+2(h H)/c)f(h)dh dt 0-
-
Of 15,000 individuals aged 18 years living in Ontario, 5,000 visited their family doctor in the past year and of these individuals 1,875 were diagnosed with lifelong depression. Assuming everyone was...
-
If A = 9 3 -5 -8 -7 01-87 2 00-765 000-4 3 0 0 0 0 -9 then det (A) =
-
To improve the effectiveness of its teaching staff, the administration of a high school offered the opportunity for all teachers to participate in a workshop. They were not required to attend;...

Study smarter with the SolutionInn App


