Consider only the transitions involving the first four energy levels for a hydrogen atom: a. How many
Question:
Consider only the transitions involving the first four energy levels for a hydrogen atom:
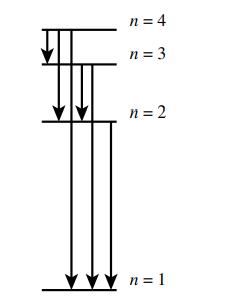
a. How many emissions are possible for an electron in the n = 4 level as it goes to the ground state?
b. Which electronic transition is the lowest energy?
c. Which electronic transition corresponds to the shortest wavelength emission?
Transcribed Image Text:
n = 4 n = 3 n = 2 n=1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Solution a The lowest energy transition is the 1S32 2S12 transition for which there are two possible ...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
A hydrogen atom has an electron in the n = 5 level. (a) If the electron returns to the ground state by emitting radiation, what is the minimum number of photons that can be emitted? (b) What is the...
-
The lowest four energy levels for atomic vanadium (V) have the following energies and degeneracies: What is the contribution to the average energy from electronic degrees of freedom for V when T =...
-
An excited hydrogen atom with an electron in the n = 5 state emits light having a frequency of 6.90 1014 s-1. Determine the principal quantum level for the final state in this electronic transition.
-
In a given week, 12 babies are born in hospital. Assume that this sample came from an underlying normal population. The length of each baby is routinely measured and is listed below (in cm): 49, 50,...
-
For a new product, sales volume in the first year is estimated to be 80,000 units and is projected to grow at a rate of 4% per year. The selling price is $ 12 and will increase by $ 0.50 each year....
-
Explain the factors that must be considered when designing a distribution strategy.
-
In a chemical laboratory, it is proposed to carry out the reaction \[ \mathrm{C}_{2} \mathrm{H}_{4}(\mathrm{~g})+\mathrm{H}_{2} \mathrm{O}(\mathrm{g}) ightarrow \mathrm{C}_{2} \mathrm{H}_{5}...
-
The April transactions of Wiset Company are described in Problem 7- 1A. In Problem 7- 1, Wiset Company completes these transactions during April of the current year (the terms of all its credit sales...
-
You are planning a trip to the United Kingdom and expect thatyou will spend 2,200 pounds. How much will your spending be in U.S.dollars if the exchange rate is .7269 pounds per dollar?
-
Tim works in Nova Scotia and is paid on a monthly basis. He is receiving a special pay (in addition to his 12 pays in the year) for a long service award. He is receiving an amount of $500 cash and he...
-
In the 1930s, electron microscopes were first used to provide structural details of human cells. If an electron microscope uses electrons having a wavelength of 0.1 nm, calculate the velocity of the...
-
A particle has a velocity that is 90.% of the speed of light. If the wavelength of the particle is 1.5 10 -15 m, calculate the mass of the particle.
-
In Exercises 1726, let Solve each matrix equation for X. A - X = 4B A = -3 -7 2 -9 5 0 and B = -5 -1 0 0 3 -4
-
A transit bus will cost $500,000 and have annual operating costs of $75,000 for 10 years. After 10 years, the expected salvage value is $50,000. At an interest rate of 4%, what is the equivalent...
-
Public utilities' balance sheets list the plant assets before the current assets. This is acceptable under which accounting principle/guideline? A large company purchases a $250 digital camera and...
-
A state highway inspector needs an estimate of the mean weight of trucks. He selects a random sample of 49 trucks passing the weighing station and finds the mean is 15.8 tons, with a standard...
-
Assume the cost to store 10,000 million BTU of natural gas is $0.05 per month. Using the current spot price, and assuming that interest rates are 1.00% p.a., is the natural gas future priced...
-
The following is the account of cash transactions of the Nari Kalayan Samittee for the year ended December 31, 2006: Receipts Amount Rs Payments Amount Rs Balance from last year 2,270 Rent 6,600...
-
Draw the most stable conformer of the following molecule CH3 CH
-
Find the inverse, if it exists, for the matrix. -1
-
Indicate whether each of these objects is chiral or achiral: (a) Golf ball (b) Baseball glove (c) Clock (d) T-shirt (e) Dress shirt (f) Automobile
-
Determine whether each of these molecules is chiral, for those that are chiral, put an asterisk at the chirality center. b) a) d) I e) )
-
Indicate whether each of these objects or molecules has a plane of symmetry: c) Ear b) Pencil a) Idealized human face I CH3 e) f) d) CH3 . Cl Br CH3 "H. CH3 "H g) h) Cl
-
Image transcription text Wind tunnel measurements of the pressure and skin friction around a NACA 2415 airfoil at 8 degrees angle of attack resulted in the following data of pressure and skin...
-
Image transcription text The following table contains load-extension data from a tensile test on a cylindrical specimen with gauge length 9mm and gauge diameter 5mm. Load-extension Data Load [KN] 0...
-
Image transcription text Systems Modelling and Analysis - Assignment 1 Due: Friday 25/08/2022 by 5:00:00 pm. To be submitted individually on Canvas and Gradescope. Part 1: Dartboard Positioning...

Study smarter with the SolutionInn App


