Most reactions occur by a series of steps. The energy profile for a certain reaction that proceeds
Question:
Most reactions occur by a series of steps. The energy profile for a certain reaction that proceeds by a two-step mechanism is
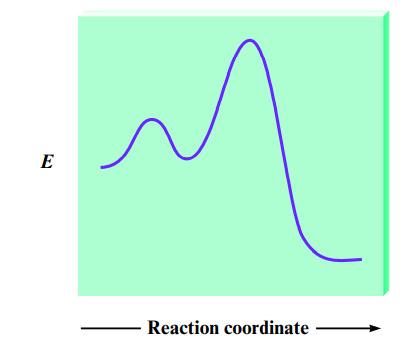
On the energy profile, indicate
a. The positions of reactants and products.
b. The activation energy for the overall reaction.
c. ΔE for the reaction.
d. Which point on the plot represents the energy of the intermediate in the two-step reaction?
e. Which step in the mechanism for this reaction is rate determining, the first or the second step? Explain.
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:




