One reason suggested for the instability of long chains of silicon atoms is that the decomposition involves
Question:
One reason suggested for the instability of long chains of silicon atoms is that the decomposition involves the transition state shown below:
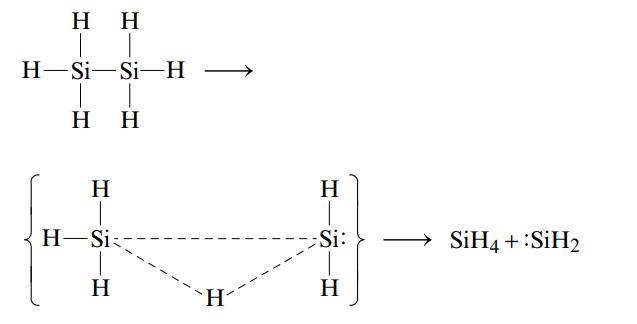
The activation energy for such a process is 210 kJ/mol, which is less than either the Si-Si or the Si-H bond energy. Why would a similar mechanism not be expected to play a very important role in the decomposition of long chains of carbon atoms as seen in organic compounds?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





