Pure zinc is to be diffused into copper by dipping copper into molten zinc at 450C. Calculate
Question:
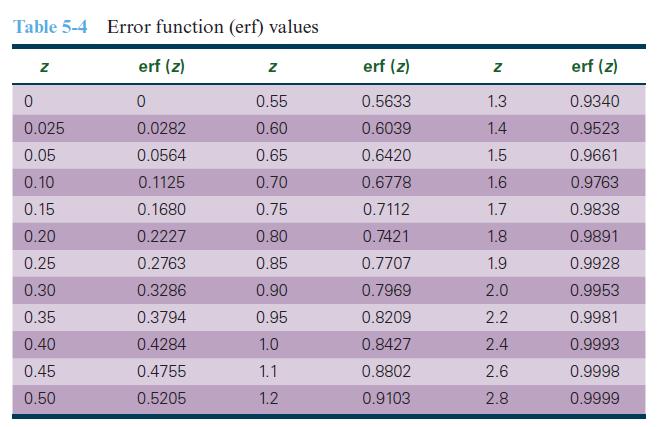
Pure zinc is to be diffused into copper by dipping copper into molten zinc at 450°C. Calculate how long it would take to obtain 10 wt% zinc at a depth of 0.5 mm beneath the copper surface. Is this commercially feasible? What practical problems might arise if we raise the temperature to 1000°C? Use Table 5-4 for error function values as needed. At 450°C, the diffusion coefficient D = 6.3 x 10-13 cm2/s.
Transcribed Image Text:
Table 5-4 Error function (erf) values erf (z) 0 0.0282 0.0564 0.1125 0.1680 0.2227 0.2763 0.3286 0.3794 0.4284 0.4755 0.5205 Z 0 0.025 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45 0.50 z 0.55 0.60 0.65 0.70 0.75 0.80 0.85 0.90 0.95 1.0 1.1 1.2 erf (z) 0.5633 0.6039 0.6420 0.6778 0.7112 0.7421 0.7707 0.7969 0.8209 0.8427 0.8802 0.9103 Z 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.2 2.4 2.6 2.8 erf (z) 0.9340 0.9523 0.9661 0.9763 0.9838 0.9891 0.9928 0.9953 0.9981 0.9993 0.9998 0.9999
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a Using the reaction kinetics equation Zn Zns Zn2xxo D 106 cm2s When the reaction rate is proportion...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Nitrogen from a gaseous phase is to be diffused into pure iron (BCC) at 700C. If the surface concentration is maintained at 0.10 wt% N, what will the concentration 1 mm from the surface be after 10...
-
Nitrogen from a gaseous phase is to be diffused into pure iron at 700C. If the surface concentration is maintained at 0.1 wt% N, what will be the concentration 1 mm from the surface after 10 h? The...
-
Nitrogen from a gaseous phase is to be diffused into pure iron at 700C. If the surface concentration is maintained at 0.1 wt% N, what will be the concentration 1 mm from the surface after 10 h? The...
-
Choose a company or product that you have been noticing or admiring. Develop a plan to advertise this product that involves the United States and at least one other country, and three forms of media....
-
Residential tenancies are much more heavily regulated than commercial tenancies, largely to protect residential tenants from being exploited by landlords. Are residential tenancies too heavily...
-
An investor has a certain amount of money available to invest now. Three alternative investments are available. The estimated profits ($) of each investment under each economic condition are...
-
In Exercises 1 to 4, it may be helpful to draw a figure such as Figure 5.5. Figure 5.5. Using the normal curve table, determine the area of the standard normal distribution that is between the...
-
Eric, your friend, received his Form W-2 from his employer (below) and has asked for your help. Erics 2018 salary was $145,000 and he does not understand why the amounts in Boxes 1, 3 and 5 are not...
-
How is the injection velocity calculated for an interplanetary flight to Mars?
-
The objective of this project is to explore applying data analytics tools to address a business question/problem, and communicate insights derived from your interpretation of your analytics results....
-
Use the diffusion data in the table below for atoms in iron to answer the questions that follow. Assume metastable equilibrium conditions and trace amounts of C in Fe. The gas constant in SI units is...
-
In ionic materials, is diffusion expected to occur at a faster rate for cations or anions? Explain.
-
An offer of payment made to fulfi ll the terms of a contract. a. anticipatory bre ach b. impossibility of performance c. material a lteration d. mitigation e. promissory not e f. specifi c...
-
Indicate whether the following statement is true, false, or uncertain, and explain your answer: If every type of water use were 100 percent nonconsumptive, the only major problems regarding the world...
-
Name the four conditions for settlement explained in this chapter. For one of them, explain a deterrent to reaching that condition and a remedy for that deterrent.
-
Imagine a world in which individuals cared only about themselves. Choose two of the following types of people and describe, in one paragraph each, how you think their behavior would differ in this...
-
Do you feel that lawyers can bring justice to those who need it while stopping short of encouraging unnecessary litigation? Explain your answer.
-
Former Microsoft employee Vern Reborn founded Eclipse Aviation in 1998. The idea was to solve a problem in the aviation industry: although private jet service is safe and convenient, it is also very...
-
The Apriori algorithm uses a generate-and-count strategy for deriving frequent itemsets. Candidate itemsets of size k + 1 are created by joining a pair of frequent itemsets of size k (this is known...
-
Briefly discuss the implications of the financial statement presentation project for the reporting of stockholders equity.
-
A typical grinding wheel is 9 in. in diameter, 1 in. thick, and weighs 6 lb. The wheel contains SiC (density of 3.2 g/cm3) bonded by a silica glass (density of 2.5 g/cm3); 5 vol% of the wheel is...
-
An electrical contact material is produced by infiltrating copper into a porous tungsten carbide (WC) compact. The density of the final composite is 12.3 g/cm3. Assuming that all of the pores are...
-
An electrical contact material is produced by first making a porous tungsten compact that weighs 125 g. Liquid silver is introduced into the compact; careful measurement indicates that 105 g of...
-
Tide PODS are a line of laundry detergent pods from Procter & Gamble under the Tide brand. The pods gained notoriety starting in 2017 when social media sites began to show people intentionally eating...
-
Marketing Management Final Project Overview Write a 2000-2500 word international consultant-like report. The report must discuss the positioning of a product or service from the country in which you...
-
identify a minimum of four relevant esports trends and data that are central to designing a successful hybrid esports event. Use recent data sets that were published in the second half of 2020 or...

Study smarter with the SolutionInn App


