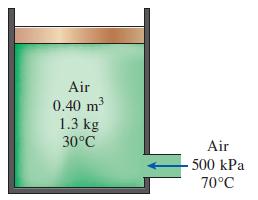
Question: A 0.40-m 3 insulated pistoncylinder device initially contains 1.3 kg of air at 30C. At this state, the piston is free to move. Now air
A 0.40-m3 insulated piston–cylinder device initially contains 1.3 kg of air at 30°C. At this state, the piston is free to move. Now air at 500 kPa and 70°C is allowed to enter the cylinder from a supply line until the volume increases by 50 percent. Using constant specific heats at room temperature, determine
(a) The final temperature
(b) The amount of mass that has entered
(c) the work done
(d) the entropy generation.
Air 0.40 3 1.3 kg m 30C Air 500 kPa 70C
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Air is allowed to enter an insulated pistoncylinder device until the volume of the air increases by ... View full answer

Get step-by-step solutions from verified subject matter experts


