A container at a pressure p and temperature T contains two substances 1 and 2, present both
Question:
A container at a pressure p and temperature T contains two substances 1 and 2, present both in liquid and gas phases. Estimate the partial pressure pA of substance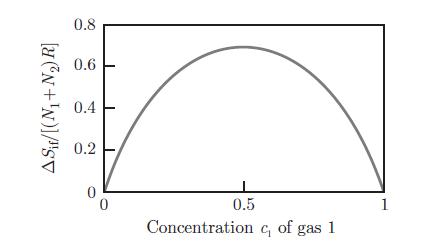 Entropy of mixing, as a function of the concentration c1 of gas 1.A in the gas phase (A = 1, 2) as a function of the concentrations c(ℓ)1 and c(ℓ)2 of substances 1 and 2 in the liquid phase. Raoult’s law relates the partial pressure PA of substance A to the saturation pressure p◦A,
Entropy of mixing, as a function of the concentration c1 of gas 1.A in the gas phase (A = 1, 2) as a function of the concentrations c(ℓ)1 and c(ℓ)2 of substances 1 and 2 in the liquid phase. Raoult’s law relates the partial pressure PA of substance A to the saturation pressure p◦A,![]() where the saturation pressure p◦A is the pressure that the pure substance A would have in the gas phase in equilibrium with the liquid phase at temperature T. Establish Raoult’s law by assuming that the liquid and gas mixtures can be treated as ideal mixtures (§ 8.5.2) and by considering that molar volumes in the liquid phase are negligible compared to molar volumes in the gaseous phase.
where the saturation pressure p◦A is the pressure that the pure substance A would have in the gas phase in equilibrium with the liquid phase at temperature T. Establish Raoult’s law by assuming that the liquid and gas mixtures can be treated as ideal mixtures (§ 8.5.2) and by considering that molar volumes in the liquid phase are negligible compared to molar volumes in the gaseous phase.
Step by Step Answer:

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet





