At sea level, water from the outlet of a river is diverted to a power plant that
Question:
At sea level, water from the outlet of a river is diverted to a power plant that operates on the principle of osmosis. A turbine is installed in the pipe that brings the river water to an osmotic membrane separating the clear water from the salt water of the sea. The sea water at the location of the membrane is assumed to have a constant low salt concentration c, i.e. c0. Because of osmosis, water is driven from the river through the turbine and then across the osmotic membrane into the sea. Just after the turbine and before the membrane the pressure is p1 = p0 − Δp. Calculate the mechanical power of the water flowing through the turbine,![]()
where v is the molar volume of water and N˙ is the number of moles per unit time flowing through the osmotic membrane. The hydrodynamics of the turbine is such that we can assume,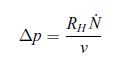
so that PW = RH N˙ 2, i.e. it is similar to the form of the Joule power for electrical heating. Use the ideal mixture relation (8.68) to determine the mechanical power PW. Since the salt concentration c is low enough, we can assume at ambient temperature that Δμ>>RTc. Show that the mechanical power is given by,![]()
where Δμ is the chemical potential drop between the river and the sea water.
Step by Step Answer:

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet





