Chemical Power An open system consists of a fluid of a single substance kept between two pistons
Question:
Chemical Power An open system consists of a fluid of a single substance kept between two pistons sliding inside a cylinder with adiabatic walls. Matter enters and exits the cylinder in two specific locations. These two matter flows generate a chemical power PC. The pressure at the entrance is p+ and the pressure at the exit is p−. The pistons exert a mechanical power PW on the fluid. Since the walls are adiabatic there is a heat transfer through convection but not through conduction, i.e. PQ = 0 (Fig. 4.12). For this open system, show that the chemical power PC generated by the matter flow can be written as,![]()
where N˙+ and N˙− are the rates of substance entering and exiting the system and h+ and h− are the molar enthalpies entering and exiting the system.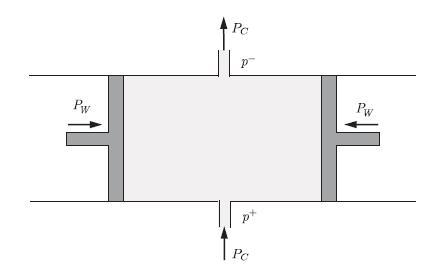
Two pistons slide in a cylinder that contains a fluid that enters and exits the system. The pressure at the entrance is p+ and the pressure at the exit is p−. The mechanical power generated by the pistons on the systemis PW and the chemical power generated by the matter flows is PC .
Step by Step Answer:

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet





