Consider the system in the figure below. Initially the gas in the cylinder is at high pressure
Question:
Consider the system in the figure below. Initially the gas in the cylinder is at high pressure Pi and the piston held in place at volume Vi with a lock. At time zero the lock is released and the gas allowed to expand, accelerating the piston of mass Mp to the left. Friction is negligible. The expansion may be idealized as an adiabatic polytropic process with ∝ = 1.4, and there is negligible leakage. The pressure on the left side of the piston may be taken as a constant P0 during the process.
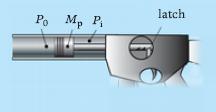
(a) Derive an expression for the velocity of the piston Ѵp as a function of the instantaneous gas volume V; the symbols defined above (and no others) will appear as parameters in your analysis.(b) Plot Ѵp versus V and P versus V for the “can-thrower” case Mp = 0.5 kg, P0 = 105 Pa, Pi = 7 × 105 Pa, Vi = 10−3 m3, covering the range for which P ≥ P0.(c) Specify the cylinder length required to produce 40 m/s when P = P0 for the canthrower, assuming the cylinder diameter is 8 cm.
Step by Step Answer:

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna





