The oxidation of methane can take place according to either one of the following reactions: When the
Question:
The oxidation of methane can take place according to either one of the following reactions: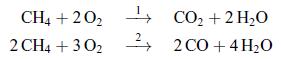
When the reactions stop at time tf because all the methane is burned, the total mass of the products (CO2, CO, H2O) is![]() Determine the initial mass of methane mCH4 (0) in terms of the total mass of the products m(tf) and the mass of water mH2O (tf). Numerical Application:
Determine the initial mass of methane mCH4 (0) in terms of the total mass of the products m(tf) and the mass of water mH2O (tf). Numerical Application:![]()
Transcribed Image Text:
CH4 +20₂ CO₂ + 2H₂O 2 CH4 +30₂22CO+ 4H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
We need to find the initial mass of methane mCH40 in terms of the total mass of the products mtf and ...View the full answer

Answered By

Alex Maina Muigai
I am a recent graduate of Maseno University, where I earned a Bachelor of Science degree in Chemical Engineering. During my studies, I developed a strong knowledge base in chemical engineering principles, including chemical reaction engineering, fluid mechanics, and thermodynamics. I also gained extensive experience in laboratory techniques and process engineering.
Since graduating, I have been working as a tutor in the field of chemical engineering. I have been providing guidance and support to students in the topics of chemical engineering, including designing chemical processes, designing experiments, and troubleshooting problems. I am also proficient in mathematics, physics, and chemistry, which I use to help my students understand the concepts of chemical engineering.
I have a passion for teaching and helping others understand complex concepts. I have a strong commitment to always providing accurate and reliable information to my students, and I strive to ensure that they have a positive learning experience. I am confident that my knowledge and experience in chemical engineering will be an asset to any student who chooses to work with me.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:
Students also viewed these Engineering questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Smart Sets manufactures headphone cases. During September 2016, the company produced 108,000 cases and recorded the following cost data: Requirements 1. Compute the cost and efficiency variances for...
-
Spencer Cook died on July 18 of the current year, leaving a gross estate of $4,600,000. Claims to be settled against that estate included funeral, administrative, and medical expenses of $180,000 and...
-
Are workpaper adjustments and eliminations entered on the parents books? The subsidiarys books? Explain.
-
The efficiency of a Carnot engine working between \(500 \mathrm{~K}\) and \(200 \mathrm{~K}\) is (a) \(80 \%\) (b) \(75 \%\) (c) \(60 \%\) (d) \(70 \%\).
-
Your company has asked you to determine the financial risks of manufacturing 6,000 units of a product rather than purchasing them from a vendor at $66.50 per unit. The production line will handle...
-
12.57 A logistic growth model for world population, f(x), in billions, x years after 1949 is f(x)= According to 1+411e -0.026x this model, when will the world population be 11 billion? According to...
-
Refer back to the beginning of this chapter to the excerpt from a Los Angeles Times article about Reed Slatkin's fraud. The article insinuates that the FBI and IRS's raiding of Slatkin's office...
-
Apply the general definition of the battery potential, to the Daniell cell ( 8.7.4) and show that it yields relation (8.108). Show that the battery potential can be written as, where - 1 zFF A VaA PA
-
Acetylene (C 2 H 2 ) can be produced through a chemical reaction between water (H 2 O) and calcium carbide (C a C 2 ): where (s) and (l) indicate whether the substance is solid or liquid. A cave...
-
Margin of error: 0.005; confidence level: 99%; p and q unknown Use the given data to find the minimum sample size required to estimate a population proportion or percentage.
-
ABC Ltd. produces a single product that sells for 75 per unit. Cost data are: (a) Variable manufacturing costs, 35 per unit. (b) Variable selling and administrative expenses, 5 per unit. (c) Fixed...
-
Assuming that the pound is worth 1. 1567 euros in Paris and 1. 4393 Swiss francs in Zurich, can Britain-based arbitrageurs make profits, given that the Swiss franc is worth 0. 8102 euros in Paris? a....
-
Assume that the Thai baht (THB) is quoted as THB 30. 2511-3987 per US$1 and that the Japanese yen () is quoted as 76. 2518-7985 per US$1. What is the cross /THB bid-ask price that the bank would...
-
Cookwell Ltd. manufactures pressure cookers with the selling price being 300 per unit. Currently the capacity utilisation is 60 per cent with a sales turnover of 18 lakh. The company proposes to...
-
Prepare a flexible budget from the following data made available in respect of a half-yearly period and forecast the working results at 70, 85, and 100 per cents of capacity when the respective sales...
-
Have you ever tried to get out of jury duty? About 25% of those called will find an excuse (work, poor health, travel out of town, etc.) to avoid jury duty. If 12 people are called for jury duty: (a)...
-
What is an insurable interest? Why is it important?
-
Obtain the expression for the isothermal compressibility from the Helmholtz energy a = a - (T,v).
-
How can the residual part of c P of a simple compressible fluid be evaluated from an an equation of state in the form P = P - (T, v)?
-
Work out the differential relation between specific entropy of a simple compressible fluid and its temperature and pressure, such that it can be evaluated with the help of c P and an equation of...
-
In what ways do global collaborations among top-tier universities contribute to advancements in various academic fields ?
-
Purdue Book Store is trying to decide on how many copies of a book to purchase from the McGraw Hill publisher at the start of a selling season. The book retails at $28 per copy at the book store, and...
-
= This numerical example illustrates mathematically the same concept shown graphically in Figure 5.16 of Williamson's text (6th edition). The economy has a representative consumer with preferences...

Study smarter with the SolutionInn App


