Suppose 2.00 mol of a diatomic gas is taken reversibly around the cycle shown in the T-5
Question:
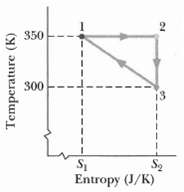
Suppose 2.00 mol of a diatomic gas is taken reversibly around the cycle shown in the T-5 diagram of Figure where S1 = 6.00 J/K and S2 = 8.00 J/K. The molecules do not rotate or oscillate. What is the energy transferred as heat Q for
(a) Path 1 ?? 2,
(b) Path 2 ?? 3 and
(c) The full cycle?
(d) What is the work W for the isothermal process? The volume V1 in state 1 is 0.200 m3. What is the volume in?
(e) State 2 and
(f) Stat 3? What is the change ΔEint for?
(g) Path 1 ?? 2,
(h) Path 1 ?? 3, and
(i) The full cycle?
(j) What is the work W for the adiabatic process?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:





