Question: The following equations are given for the liquid-phase activity coefficients of the water (W)-acetic acid (A) system. Find the dew point and bubble point of
The following equations are given for the liquid-phase activity coefficients of the water (W)-acetic acid (A) system.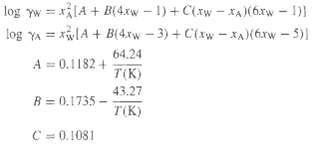 Find the dew point and bubble point of a mixture of composition xw = 0.5, xA = 0.5 at 1 atm. Flash the mixture at a temperature halfway between the dew point and the bubblepoint.
Find the dew point and bubble point of a mixture of composition xw = 0.5, xA = 0.5 at 1 atm. Flash the mixture at a temperature halfway between the dew point and the bubblepoint.
log yw = x[A + B(4xw 1) + C(xw - xA )(6xw - 1) log YA = xlA + B(4.xw - 3) + C(xw - X)(6xw - 5)) 64.24 A = 0.1182 + T(K) 43.27 R = B = 0.1735 - T(K) C= 0.1081
Step by Step Solution
3.32 Rating (170 Votes )
There are 3 Steps involved in it
The RachfordRice flash equations can be used from Table 44 where z W 05 and z A 05 and the modified Raoults law is Antoine vapor pressure equations ar... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (122).docx
120 KBs Word File


