Find the bubble-point and dew-point temperatures of a mixture of 0.4 mole fraction toluene (1) and 0.6
Question:
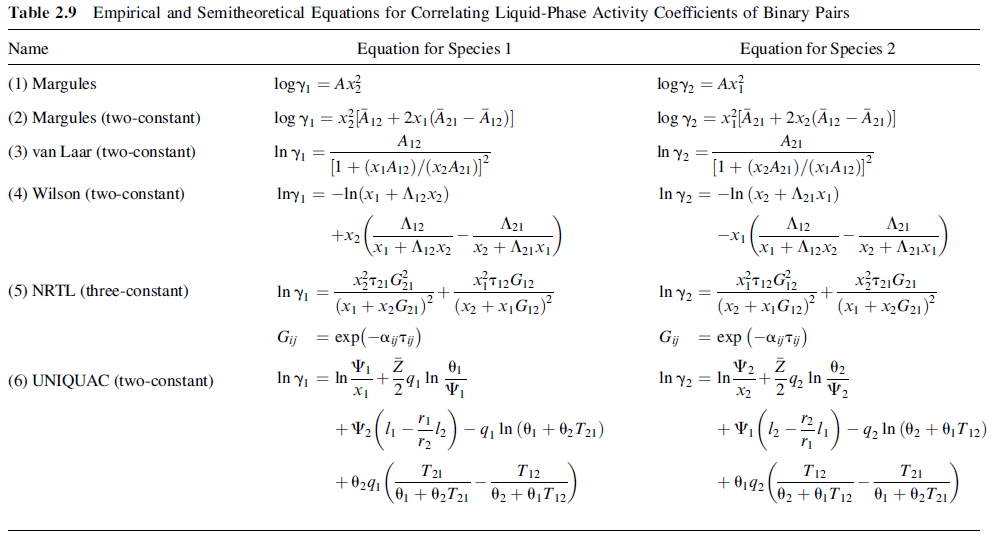
Find the bubble-point and dew-point temperatures of a mixture of 0.4 mole fraction toluene (1) and 0.6 mole fraction n-butanol (2) at 101.3kPa. The K-values can be calculated from (2-72), the modified Raoult's law, using vapor-pressure data, and γl and γz from the van Laar equation of Table 2.9 withAI2 = 0.855 and A2l = 1.306. If the same mixture is flashed at a temperature midway between the bubble point and dew point, and 101.3kPa, what fraction is vaporized, and what are the compositions of the two phases?
Transcribed Image Text:
Table 2.9 Empirical and Semitheoretical Equations for Correlating Liquid-Phase Activity Coefficients of Binary Pairs Equation for Species 2 Name Equation for Species 1 logyi = Ax logy = Ax} (1) Margules x¡[Ã21 + 2x2 (Ã12 – Ā21)] log yi = xA 12 + 2x1(Ã21 – Ā12)] (2) Margules (two-constant) log y2 = A12 A21 In y, = [1 + (x1A12)/(x2A21)] Iny, = -In(x1 + A12x2) In y2 = [1+ (x2A21)/(x1A12)J² In y2 = -In (x2 + A21X1) (3) van Laar (two-constant) (4) Wilson (two-constant) A12 A21 A12 A21 +x2 x1 + Aj2x2 x2 + A21X1, -x1 \x1 + A12x2 x2 + A21×1, xiT12G12 (x2 + X¡G12)? X3721G21 In y (5) NRTL (three-constant) In y2 = (x1 +X2G21)? (x2 + X¡G12) (x1 + x,G21)² exp(-ajty) = exp (-ajTj) Gij Gij 02 In y, = In X1 In y2 = In- X2 In 2 In (6) UNIQUAC (two-constant) +v (4-) -4, la (0, + 0,T12) +V2 (1 -2) - q In (0, + 02T21) - q2 In (02 + 0, T 12) r2 T21 01 + 02T21 T12 T12 T21 +0291 + 0,92 02 + 0,T12 01 + 02T21. 02 + 0,T12,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
The RachfordRice flash equations can be used from Table 44 The modified Raoults law is Antoine vapor pressure in torr equations are obtained by fittin...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The radius, r, of a sphere can be calculated from its surface area, s, by: r = s/x/2 The volume, V, is given by: V = 4r3/3 Determine the volume of spheres with surface area of 50, 100, 150, 200, 250,...
-
Using vapor pressure data from Exercises 4.6 and 4.8 and the enthalpy data provided below: (a) Construct an h-x-y diagram for the benzene-toluene system at 1 atm (101.3 kPa) based on the use of...
-
Describe how a brand price elasticity can be calculated from the results of a sales experiment.
-
Write a static method lg() that takes an int argument n and returns the largest integer not larger than the base-2 logarithm of n. Do not use the Math library.
-
On January 10, 2012, Kuril Ltd. sold merchandise on account to R. James for $48,000, terms n/30. The merchandise originally cost $32,000. On February 1, R. James gave Kuril a five-month, 7% note in...
-
Draw and annotate a class hierarchy that represents various types of sales transactions in a store (cash, credit, etc.). Show what characteristics would be represented in the various classes of the...
-
An alternative to using \(d=1 / u\) in a binomial model is to use the available degree of freedom by setting \(p=1 / 2\). (a) Let \(p=1 / 2\), and find the values of \(u\) and \(d\) that satisfy the...
-
On February 1, the Miro Company needs to purchase some office equipment. The company is presently short of cash and expects to be short for several months. The company treasurer has indicated that he...
-
Below are extracted information for XYZ Limited for its first - year account ending 3 1 December 2 0 2 1 : Equipment ( NCA ) 2 0 , 0 0 0 Utilities Expense 2 , 1 0 0 Cash 5 , 0 0 0 Accounts Receivable...
-
In 1913, how long did the average worker stay with the plant? What was the average tenure of a worker? Assume the one-millionth vehicle was produced in 1916 at a cost of $8084 (in 2013 US$). By how...
-
The following equations are given for the liquid-phase activity coefficients of the water (W)-acetic acid (A) system. Find the dew point and bubble point of a mixture of composition xw = 0.5, xA =...
-
(a) For a liquid solution having a molar composition of ethyl acetate (A) of 80% and ethyl alcohol (E) of 20%, calculate the bubble-point temperature at 101.3kPa and the composition of the...
-
How may debt of the corporation affect a shareholder's basis in an S corporation?
-
What can public health communicators learn from the private sector about the perspective of the consumer?
-
Describe the distinctions among theoretical methods, practice strategies, and activities or channels.
-
What are the differences between sex and gender?
-
What is the current taken by the display connected in Fig. 3.12, when the digit 3 is showing? to 7-segment display pins 3,8 pin 7, seg. a pin 6, seg. b pin 4, seg. c pin 2, seg. d < pin 1, seg. e pin...
-
A student builds an mbed-based system. To one port he connects the circuit of Fig. 3.17A, using LEDs of the type used in Fig. 3.4, but is then disappointed that the LEDs do not appear to light when...
-
Assume that an exploratory well is drilled and hydrocarbons are indicated. However, there is substantial uncertainty regarding whether the oil or gas is sufficient to justify recognition of proved...
-
What services are provided by the provincial and territorial governments?
-
Define both OEFs and CEFs.
-
Discuss the advantages and disadvantages of the available correlations for estimating binary-pair mass-transfer coefficients for columns with random (dumped) and structured packings.
-
Does the rate-based model account for the bulk-flow effect in mass transfer?
-
What are component-coupling effects in mass-transfer-rate equations?
-
After you've completed this week's assigned reading, watch the video below. Next, respond to the Discussion questions underneath. Your first post should answer those questions and should be made by...
-
Factorize (x4 - 20x + 100)
-
Paragraph discussing food insecurity in the United States. What agencies in the federal government play an important role in ameliorating this situation? What groups of people are at particular risk?

Study smarter with the SolutionInn App


