The Lucas test is used to check for the presence of an alcohol functional group in an
Question:
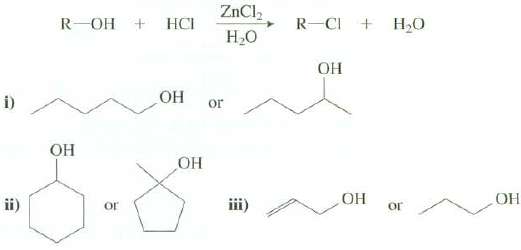
The Lucas test is used to check for the presence of an alcohol functional group in an unknown compound. The test reaction is shown in the following equation:
Smaller alcohols are soluble in the strongly acidic solution, but the corresponding chlorides are not. A positive test is indicated by the formation of an insoluble layer of the alkyl chloride.
(a) Explain why the reaction condition favor an SN1 mechanism.
(b) Which of these alcohols reacts more rapidly with HCl and ZnCl2 inH2O?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





