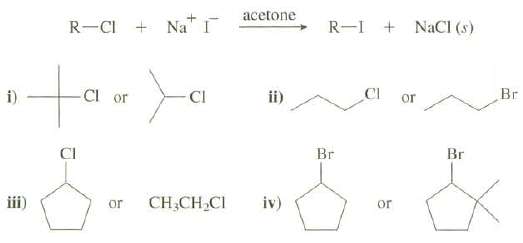
The reaction of an alkyl chloride (or bromide) with sodium iodide in acetone proceeds according to the
Question:
The reaction of an alkyl chloride (or bromide) with sodium iodide in acetone proceeds according to the following equation:
Sodium iodide is soluble in acetone, whereas both sodium chloride and sodium bromide are insoluble, so the appearance of a precipitate is a positive test for the presence of an alkyl chloride or bromide.
(a) Explain why these conditions favor the SN2 mechanism.
(b) Which of these halides would give a precipitate more rapidly when reacted with NaI inacetone?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





