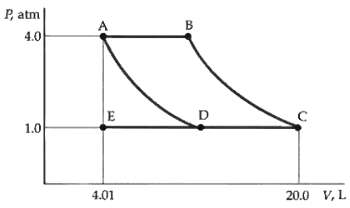
The PV diagram in figure represents 3 mol of an ideal monatomic gas. The gas is initially
Question:
The PV diagram in figure represents 3 mol of an ideal monatomic gas. The gas is initially at point A. The paths AD and BC represent isothermal changes. If the system is brought to point C along the path AEC, find
(a) The initial and final temperatures,
(b) The work done by the gas, and
(c) The heat absorbed by the gas.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:





