Question: We remarked in Impact 19.2 that the particle in a sphere is a reasonable starting point for the discussion of the electronic properties of spherical
We remarked in Impact 19.2 that the particle in a sphere is a reasonable starting point for the discussion of the electronic properties of spherical metal Nan particles. Here, we justify eqn 9.54, which shows that the energy of an electron in a sphere is quantized.
(a) The Hamiltonian for a particle free to move inside a sphere of radius R is
H = - h / 2m ∆2 Show that the Schrödinger equation is separable into radial and angular components. That is, begin by writing Ψ(r, θ, Φ) = X(r) Y (θ, Φ), where X(r) depends only on the distance of the particle away from the centre of the sphere, and Y (θ, Φ) is a spherical harmonic. Then show that the Schrödinger equation can be separated into two equations, one for X, the radial equation, and the other for Y, the angular equation:
clip_image001.emz"
o:title="" blacklevel="3932f" grayscale="t"/>
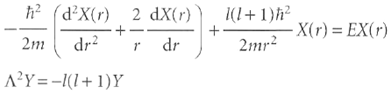
You may wish to consult further information 10.1 for additional help.
(b) Consider the case 1= 0. Show by differentiation that the solution of the radial equation has the form
X (r) = (2πR) -1/2 sin (nπr/R)/r
(c) Now go on to show that the allowed energies are given by:
En = n2h2 / 8mR2
This result for the energy (which is eqn 9.54 after substituting m, for m) also applies when l ≠ O.
(d'x(r) 2 dX(r) ) 2mdrr dr i(1+1) 2mr x( r) = EX(r) A?Y=-1(1+1)Y
Step by Step Solution
3.51 Rating (168 Votes )
There are 3 Steps involved in it
a In the sphere the Schrdinger equation is 1 2 2m ar h 2m yax 2 a where A is an operator that contains derivatives with respect to and only Let r00 Xr... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
57-C-PC-S (73).docx
120 KBs Word File


