Question: Well see in the next chapter that alkyl halides react with nucleophiles to give substitution products by a mechanism that involves inversion of stereochemistry at
We’ll see in the next chapter that alkyl halides react with nucleophiles to give substitution products by a mechanism that involves inversion of stereochemistry at carbon: Draw the reaction of (S)-2-bromobutane with HS– ion to yield 2-butanethiol, CH3CH2CH (SH) CH3. What is the stereochemistry of the product?
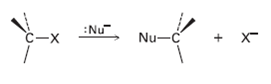
:Nu" Nu-C + X- C-X
Step by Step Solution
3.43 Rating (169 Votes )
There are 3 Steps involved in it
HS CHCH3 SO Br ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-S (197).docx
120 KBs Word File


