Question: When a system is taken from state i to state f along path iaf in Figure Q = 50 cal and W - 20 cal.
When a system is taken from state i to state f along path iaf in Figure Q = 50 cal and W - 20 cal. Along path ibf, Q = 36 cal.(a) What is W along path ibf?(b) If W = -13 cal for the return path fi, what is Q for this path?(c) If Eint,i = 10 cal, what is Eint,f? If Eint,b = 22 cal, what is Q for(d) Path ib and(e) Pathbf?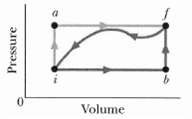
Volume Pressure
Step by Step Solution
3.41 Rating (176 Votes )
There are 3 Steps involved in it
a The change in internal energy AEint is the same for path iaf ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-F-L (442).docx
120 KBs Word File


