Question: Why is the 0.1 M HCl | 0.1 M KCl junction potential of opposite sign and greater magnitude than the 0.1 M NaCl | 0.1
Why is the 0.1 M HCl | 0.1 M KCl junction potential of opposite sign and greater magnitude than the 0.1 M NaCl | 0.1 M KCl potential in Table 14-2?
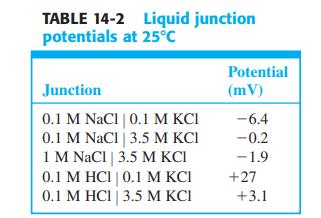
TABLE 14-2 Liquid junction potentials at 25C Potential Junction (mV) 0.1 M NaCl | 0.1 M KCI 0.1 M NaCl | 3.5 M KCI 1 M NaCl | 3.5 M KCI 0.1 M HCI | 0.1 M KCI 0.1 M HCI | 3.5 M KCI -6.4 -0.2 -1.9 +27 +3.1
Step by Step Solution
3.42 Rating (171 Votes )
There are 3 Steps involved in it
H has greater mobility than K The HC1 side of the HC1 KC1 junction will be ne... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2039).docx
120 KBs Word File


