Question: Why do the liquid junctions potentials 0.1 M HCl | 0.1 M KCl and 0.1 M NaOH | 0.1 M KCl have opposite signs in
Why do the liquid junctions potentials 0.1 M HCl | 0.1 M KCl and 0.1 M NaOH | 0.1 M KCl have opposite signs in Table 15-2? Why is the junction potential for 0.1 M NaOH | 0.1 M KCl so much more negative than 0.1 M NaOH | KCl (saturated)?
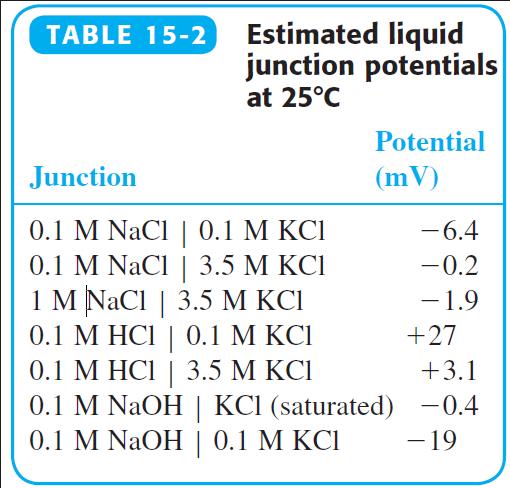
TABLE 15-2 Estimated liquid junction potentials at 25C Potential Junction (mV) 0.1 M NaCl | 0.1 M KCI 0.1 M NaCl | 3.5 M KCI 1 M NaCl | 3.5 M KCI 0.1 M HCI | 0.1 M KCI 0.1 M HCI | 3.5 M KCI 0.1 M NaOH | KCI (saturated) -0.4 0.1 M NaOH | 0.1 M KCI -6.4 | -0.2 -1.9 | +27 +3.1 -19
Step by Step Solution
3.56 Rating (170 Votes )
There are 3 Steps involved in it
Answer The liquid junction potentials 01 M HCl 01 M KCl and 01 M NaOH 01 M KCl have opposite signs b... View full answer

Get step-by-step solutions from verified subject matter experts


