Question: (a) For a liquid solution having a molar composition of ethyl acetate (A) of 80% and ethyl alcohol (E) of 20%, calculate the bubble-point temperature
(a) For a liquid solution having a molar composition of ethyl acetate (A) of 80% and ethyl alcohol (E) of 20%, calculate the bubble-point temperature at 101.3kPa and the composition of the corresponding vapor using (2-72) with vapor pressure data and the van Laar equation of Table 2.9 with AAE = 0.855, AEA = 0.753.
(b) Find the dew point of the mixture.
(c) Does the mixture form an azeotrope? If so, predict the temperature andcomposition.
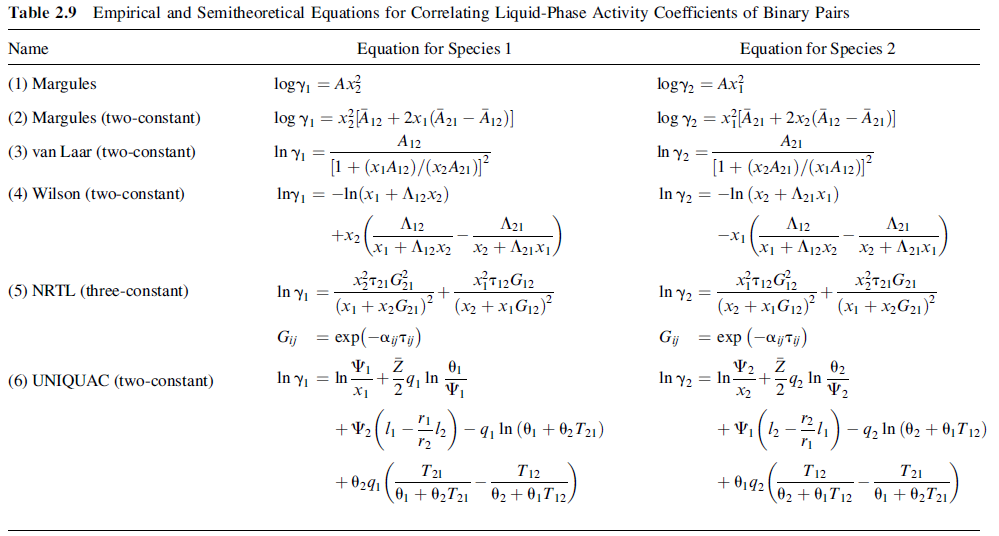
Table 2.9 Empirical and Semitheoretical Equations for Correlating Liquid-Phase Activity Coefficients of Binary Pairs Equation for Species 2 Name Equation for Species 1 logyi = Ax logy = Ax} (1) Margules x[21 + 2x2 (12 21)] log yi = xA 12 + 2x1(21 12)] (2) Margules (two-constant) log y2 = A12 A21 In y, = [1 + (x1A12)/(x2A21)] Iny, = -In(x1 + A12x2) In y2 = [1+ (x2A21)/(x1A12)J In y2 = -In (x2 + A21X1) (3) van Laar (two-constant) (4) Wilson (two-constant) A12 A21 A12 A21 +x2 x1 + Aj2x2 x2 + A21X1, -x1 \x1 + A12x2 x2 + A211, xiT12G12 (x2 + XG12)? X3721G21 In y (5) NRTL (three-constant) In y2 = (x1 +X2G21)? (x2 + XG12) (x1 + x,G21) exp(-ajty) = exp (-ajTj) Gij Gij 02 In y, = In X1 In y2 = In- X2 In 2 In (6) UNIQUAC (two-constant) +v (4-) -4, la (0, + 0,T12) +V2 (1 -2) - q In (0, + 02T21) - q2 In (02 + 0, T 12) r2 T21 01 + 02T21 T12 T12 T21 +0291 + 0,92 02 + 0,T12 01 + 02T21. 02 + 0,T12,
Step by Step Solution
3.28 Rating (154 Votes )
There are 3 Steps involved in it
The RachfordRice flash equations can be used from Table 44 The modified Raoults law from Eq 272 is Antoine vapor pressure in torr equations are obtain... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (124).docx
120 KBs Word File


