Question: A two-stage process is used to separate H 2 S from a gas containing 96% H and 4?? H 2 S by volume. ?The H
A two-stage process is used to separate H2S from a gas containing 96% H and 4?? H2S by volume. ?The H2S is absorbed in a solvent, which is then regenerated by air in a stripping column. The Henry?s law constant for the absorption of H2S in the solvent at 0?C is 22 atm/mole fractions
(a) Briefly explain in your own words the functions of the three process units. Include in your explanation the purpose of the air in the stripper and the reason the stripper operates at a higher temperature than the absorber.
(b) Calculate the molar flow rate of pure solvent and the volumetric flow rate of the gas at 04, neglecting evaporation of solvent in both columns. (See flowchart.)
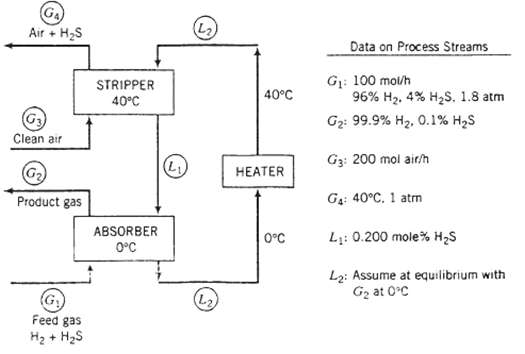
GA Air + HS Clean air Product gas (G) Feed gas H + HS STRIPPER 40C ABSORBER 0C (L) (L) (L) 40C HEATER 0C Data on Process Streams G: 100 mol/h 96% H, 4% HS, 1.8 atm G: 99.9% H, 0.1% HS G3: 200 mol air/h G: 40C. 1 atm L: 0.200 mole% HS L: Assume at equilibrium with G at 0C
Step by Step Solution
3.25 Rating (174 Votes )
There are 3 Steps involved in it
Basis Given feed rates G1 100 molh 096 H 004 H S satd 18 atm absorber 0C 12 n molh X3 mol H... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (325).pdf
180 KBs PDF File
13-E-C-E-C-P (325).docx
120 KBs Word File


