Question: At a point in an ammonia absorber using water as the absorbent and operating at 101.3 kPa and 20C, the bulk gas phase contains 10
At a point in an ammonia absorber using water as the absorbent and operating at 101.3 kPa and 20°C, the bulk gas phase contains 10 vol% NH3. At the interface, the partial pressure of NH3 is 2.26 kPa. The concentration of the ammonia in the body of the liquid is 1 wt%. The rate of ammonia absorption at this point is 0.05 krnovh-m2.
(a) Given this information and the equilibrium curve in Figure, calculate X, Y, Yr, XI, X*, Y*, Ky, Kx, ky, and kx.
(b) What percent of the mass-transfer resistance is in each phase?
(c) Verify for these data that 1/Ky = l/ky +H'/kx.
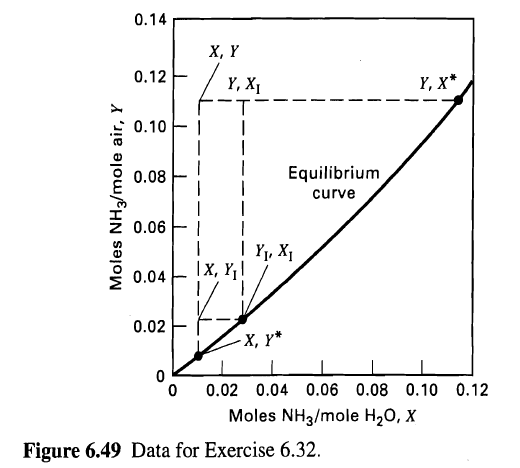
0.14 , Y 0.12 , X* Y, X1 0.10 F I Equilibrium 0.08 curve 0.06 | Yy, X1 x, ,! 0.04 0.02 , * 0.02 0.04 0.06 0.08 0.10 0.12 Moles NH3/mole H20, X Figure 6.49 Data for Exercise 6.32. Moles NH3/mole air, Y
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
a At the point as shown in Fig the mole ratios in the bulk are X 1179918 00107 and Y 0109 0111 At th... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (237).docx
120 KBs Word File


