Question: Calculate the formal charges on the atoms shown inred. (a) (CH3)2OBF3 (b) H2C-NEN: (c) H2C=N=N: (d) :=-: (e) H (f) - CH
Calculate the formal charges on the atoms shown inred.
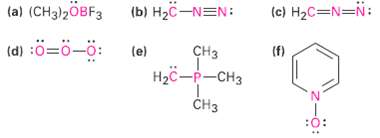
(a) (CH3)2OBF3 (b) H2C-NEN: (c) H2C=N=N: (d) :=-: (e) H (f) - CH
Step by Step Solution
★★★★★
3.48 Rating (161 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

To save space molecules are shown as linebond structures with lone pairs rather than as electrondo... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
22-C-O-A-B-R (82).docx
120 KBs Word File


