Question: Draw as many resonance structures as you can for the following species. Adding appropriate formal charger to each: (a) Nitromethane, (b) Ozone, (c) Diazomethane, :0:
Draw as many resonance structures as you can for the following species. Adding appropriate formal charger to each:
(a) Nitromethane,
(b) Ozone,
(c) Diazomethane,
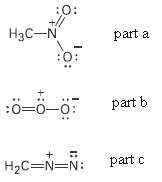
:0: +// H3C-N part a :0: part b :0=0-0: part c H2C=N=N: :o:
Step by Step Solution
3.33 Rating (174 Votes )
There are 3 Steps involved in it
a c 0 HC... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-A-B-R (103).docx
120 KBs Word File


