Question: Indicate whether these compounds are weaker or stronger acids than water (the K a for water is 1.8 x 10 ?16 ; the p K
Indicate whether these compounds are weaker or stronger acids than water (the Ka for water is 1.8 x 10?16; the pKa is 15.74):
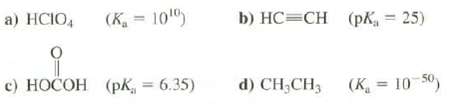
(K=100) a) HCIO4 c) HOCOH (PK = 6.35) b) HCCH (pK = 25) d) CH3CH (K=10-50)
Step by Step Solution
3.30 Rating (174 Votes )
There are 3 Steps involved in it
The acidity constant K a is a measure of the acidic strength of a ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-A-B-R (7).docx
120 KBs Word File


