The data in the following table give the solubility of silver chloride in various aqueous solutions at
Question:
The data in the following table give the solubility of silver chloride in various aqueous solutions at 25°C. Show that these data can be plotted on the same ln Ks versus √I curve as used in Illustration 13.2-3.
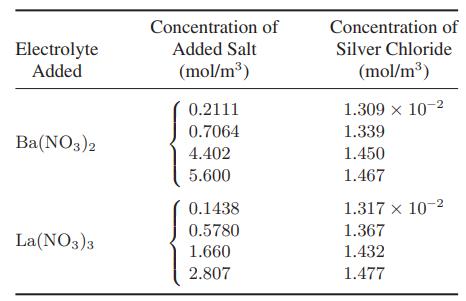
Illustration 13.2-3.
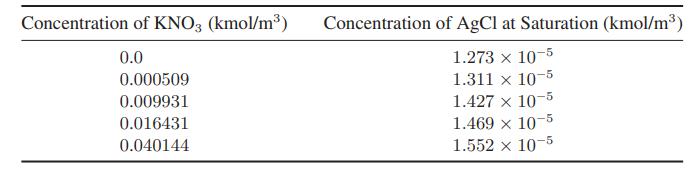
Calculation of the Solubility Product from Solubility Data The following data give the solubility of silver chloride in aqueous solutions of potassium nitrate at 25°C:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





