An important reaction in organic chemistry is that of organic halides with hydroxide ion to form alcohols.
Question:
An important reaction in organic chemistry is that of organic halides with hydroxide ion to form alcohols. You are interested in learning about the impact of temperature on the rate of this kind of reaction, so you decide to determine the Arrhenius parameters for one such reaction.
The rate constant for the second-order reaction between bromoethane and hydroxide ions in water, C2H5Br(aq) + OH2(aq) → C2H5OH(aq) + Br2(aq), was measured at several temperatures, with the results shown here: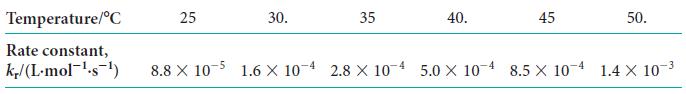
Determine the activation energy of the reaction.
ANTICIPATE Activation energies of organic reactions are typically between 10 and 100 kJ · mol–1, so you should expect a value within that range.
PLAN Activation energies are found from the Arrhenius equation (Eq. 1). Plot ln kr against 1/T, with T in kelvins. Because the slope is equal to 2Ea/R, to find the activation energy multiply the slope of the graph by 2R with R = 8.3145 J · K–1 · mol–1. A spreadsheet, curve-fitting program, or graphing calculator is very useful for this type of calculation.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





