For each compound below, identify all lone pairs and indicate whether each lone pair is localized or
Question:
a.
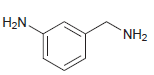
b.
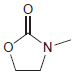
c.
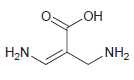
d.
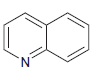
e.
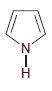
f.
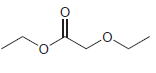
Transcribed Image Text:
H2N `NH2 z.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a b c d e f H IZ delo...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw all lone pairs on each of the oxygen atoms in the compounds below. Before doing this, review in the following table, and then come back to these problems. Try to identify all lone pairs without...
-
Draw all lone pairs on each of the nitrogen atoms in the compounds below. First, review in the following table, and then come back to these problems. Try to identify all lone pairs without having to...
-
Each of the following compounds contains both oxygen and nitrogen atoms. Identify all lone pairs in each of the following compounds: a. b. c. d. e. f. N. O=C=N
-
Compute the indicated quantities for the given homomorphism. Ker () for : S 3 Z 2 in Example 13.3 Data from Example 13.3 Let S n be the symmetric group on n letters, and let : S n Z 2 be defined by...
-
You have been hired by First Citizens Bank as a financial analyst. One of your first job assignments is to analyze the present financial condition of Bradley Stores, Incorporated. You are provided...
-
The amount of air resistance that acts on a wingsuit flyer (and a flying squirrel) depends on the flyers (a) area. (b) speed. (c) area and speed. (d) acceleration.
-
We have seen that we can use a bubble plot to show a third quantitative variable on a scatterplot. Another way to show three quantitative variables together is to use a 3-dimensional scatterplot,...
-
Suppose you get a job at MobileTV, a small manufacturer of TV sets installed in cars and boats. Business has declined recently, foreign rivals from emerging markets are increasing competition, and...
-
A company plans to make four annual deposits of $ 3 , 7 5 0 each to a special building fund. The fund s assets will be invested in mortgage instruments expected to pay interest at 1 2 % on the fund s...
-
Consider a homogeneous right circular cylinder of length L, radius R, and specific gravity SG = 0.5, floating in water (SG = 1) with its axis horizontal. Show that the body is stable if L/R > 2.0....
-
Use LHpitals rule lim [f(x)/g(x)] x 0 = lim [df(x)dx/dg(x)/dx] x 0 to show that the expression derived for Pf in part b of Example problem 1.1 hane the correct limit as y 0.
-
Nicotine is a toxic substance present in tobacco leaves. There are two lone pairs in the structure of nicotine. In general, localized lone pairs are much more reactive than delocalized lone pairs....
-
Why are Fe-Si alloys used in magnetic applications grain oriented?
-
Suppose demand and supply are given by Qx=14 (1/2)P and Q = (1/4)Px 1 Instructions: Enter your responses rounded to the nearest whole number. a. Determine the equilibrium price and quantity. Show the...
-
Summarize in different words the the following Over one billion people around the world live on less than $1 a day. In 2006, the median income for an individual in the United States was over $75 per...
-
Explain tonicity and how plant and animal cells deal with varying tonicity in their environments .
-
Applying a Theorist ( J ane Addams, 'Utilization of Women in City Government') to a current piece of Pop Culture Using the ideas from a specific theorist, first concisely elaborate the core ideas...
-
If a firm's production process is Q = 0.8L2 -0.2L, and labor costs $95 per worker and output sells for $5, how much labor should the firm hire?
-
Find an equation for graph. 2 2 4 3 3
-
Imagine a sound wave with a frequency of 1.10 kHz propagating with a speed of 330 m/s. Determine the phase difference in radians between any two points on the wave separated by 10.0 cm.
-
Outline all steps in a mechanism showing how 2, 3-dimethyl-2-butanol is formed in the acid-catalyzed hydration of 3, 3-dimethyl-1-butene.
-
Alkyl halides add to alkenes in the presence of AlCl3; yields are the highest when tertiary halides are used. Predict the outcome of the reaction of tert-pentyl chloride (1-chloro-2,...
-
Explain the stereochemical results observed in this catalytic hydrogenation. (You may find it helpful to build hand-held molecular models.) CH2 CH CH3 CH Pt catalyst CH3 HOAc 170%) 11 (30%)
-
Bronchial alveolar lavage and four endobronchial biopsies were performed and submitted to the insurance carrier as follows: 31625 x 4 units, 31624-51. Is this claim coded appropriately?
-
What goals are used to measure labor and management partnerships?
-
1. A Supervisor email you a question. 2. Potential client comes to your office. He has $40000 in wages on W-2. He also listed $30000 of charitable contributions, mostly clothing, on a single piece of...

Study smarter with the SolutionInn App


