Question: For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: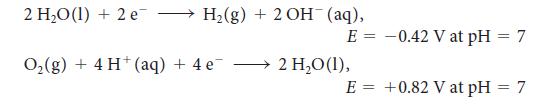 A sample of manganese of mass 4.9 g was produced from a manganese nitrate aqueous solution when a current of 350 mA was passed for 13.7h. What is the oxidation number of manganese in the manganese nitrate?
A sample of manganese of mass 4.9 g was produced from a manganese nitrate aqueous solution when a current of 350 mA was passed for 13.7h. What is the oxidation number of manganese in the manganese nitrate?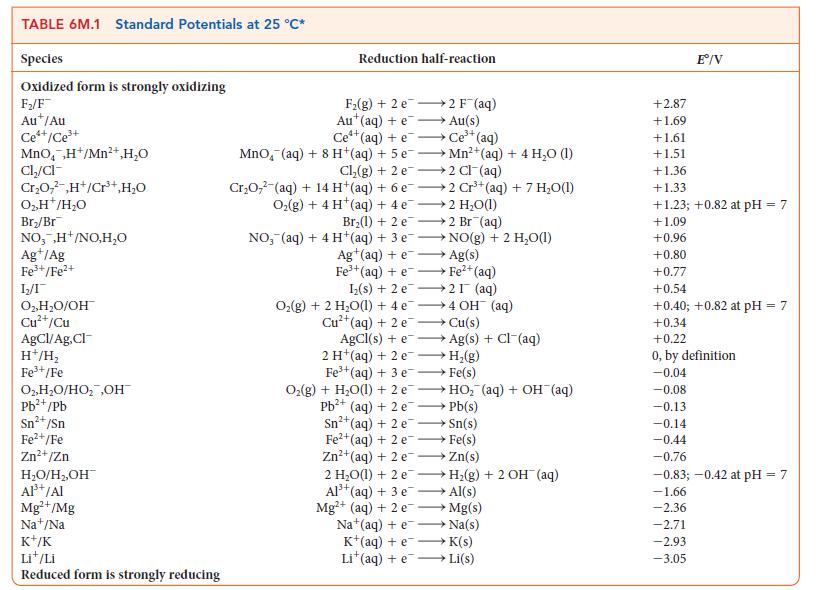
2 HO (1) + 2 e H(g) + 2 OH (aq), E = O(g) + 4 H+ (aq) + 4e 2 HO (1), E = -0.42 V at pH = 7 +0.82 V at pH = 7
Step by Step Solution
3.47 Rating (163 Votes )
There are 3 Steps involved in it
To find the oxidation number of manganese in manganese nitrate MnNO32 we can use the information giv... View full answer

Get step-by-step solutions from verified subject matter experts


