Question: For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: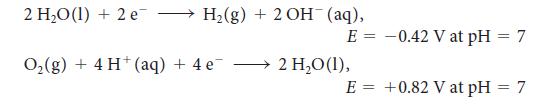 Three electrolytic cells containing solutions of CuNO3, Sn(NO3)2, and Fe(NO3)3, respectively, are connected in series. A current of 3.5 A is passed through the cells until 6.10 g of copper has been deposited in the first cell.
Three electrolytic cells containing solutions of CuNO3, Sn(NO3)2, and Fe(NO3)3, respectively, are connected in series. A current of 3.5 A is passed through the cells until 6.10 g of copper has been deposited in the first cell.
(a) What masses of tin and iron are deposited?
(b) For how long did the current flow?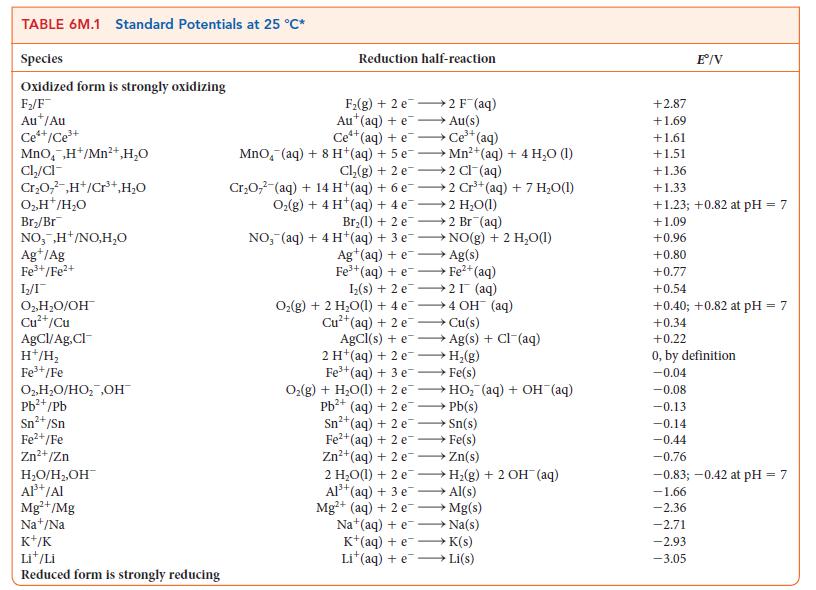
2 HO(1) + 2 e H(g) + 2 OH (aq), E = O(g) + 4 H (aq) + 4 e 2 HO (1), E = -0.42 V at pH = 7 +0.82 V at pH = 7
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
a Cuaq e Cu s 1 mol Cu 1 mole 96500 C Q 610g Cu 6355g Cu 1 mol ... View full answer

Get step-by-step solutions from verified subject matter experts


