Question: For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: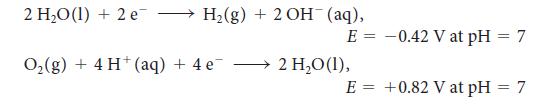
(a) When a current of 324 mA is used for 15 h, what volume (measured in liters at 298 K and 1.0 atm) of fluorine gas can be produced from a molten mixture of potassium and hydrogen fluorides?
(b) With the same current and time period, how many liters of oxygen gas at 298 K and 1.0 atm can be produced from the electrolysis of water?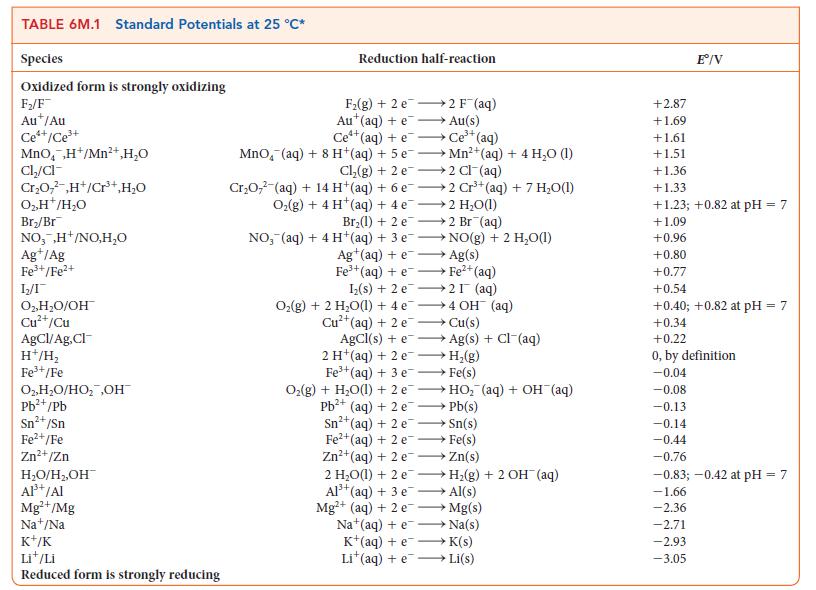
2 HO (1) + 2 e H(g) + 2OH(aq), O(g) + 4 H(aq) + 4e E = -0.42 V at pH = 7 2 HO (1), E = +0.82 V at pH = 7
Step by Step Solution
3.50 Rating (147 Votes )
There are 3 Steps involved in it
To find the volumes of fluorine gas and oxygen gas produced through electrolysis based on a given cu... View full answer

Get step-by-step solutions from verified subject matter experts


