In a study to see how closely gaseous ammonia obeys Boyles law, several volume measurements were made
Question:
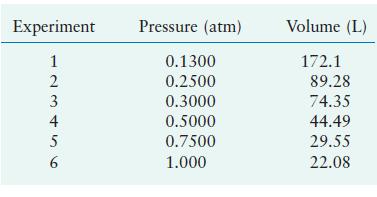
In a study to see how closely gaseous ammonia obeys Boyle’s law, several volume measurements were made at various pressures using 1.0 mol of NH3 gas at a temperature of 0°C. Using the results listed below, calculate the Boyle’s law constant for NH3 at the various pressures.
Transcribed Image Text:
Experiment 123456 2 4 5 Pressure (atm) 0.1300 0.2500 0.3000 0.5000 0.7500 1.000 Volume (L) 172.1 89.28 74.35 44.49 29.55 22.08
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To determine how closely NH3 gas follows Boyles law under these conditions we calculate the ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
In a study to examine the utility of using ammonia gas to sanitize animal feeds, researchers inoculated corn silage with a strain of Salmonella. Next, two petri dishes of 5 g of contaminated feed...
-
In a study to predict temperature from air pressure in a piston-cylinder device, 19 measurements were made of temperature in ¦f (y) and air pressure in psi (x). three models were fit: the...
-
In Pissarides's view of labor markets (job matching model), the focus is on finding a good ________. a. level of TFP (A) b. supply of money (M). c. match d. Wage
-
For the beam and loading shown, (a) Draw the shear and bending-moment diagrams, (b) Determine the maximum absolute values of the shear and bending moment. 150 lb 100 I 100 Ib 12 lvin. 12 lbvin. tra...
-
Carry out a posterior analysis of the control device problem. That is, decide whether the engineers should be sent, and find the expected monetary value associated with either sending or not sending...
-
In July 2008, Brian, Dale, and Sandra Allen signed a contract with East Resources, Inc., concerning 148 acres of the Allens property. East wanted to develop and exploit the oil and gas resources...
-
The condensed single-step income statement for the year ended December 31, 2014, of Conti Chemical Company, a distributor of farm fertilizers and herbicides, follows. Selected accounts from Conti...
-
Explain the relationship and the difference between online analytical processing systems and customer relationship management systems within a business intelligence program.?
-
A sample of hydrogen gas (H 2 ) has a volume of 8.56 L at a temperature of 0C and a pressure of 1.5 atm. Calculate the moles of H 2 present in this gas sample.
-
* The formate ion, \(\mathrm{CHO}_{2}{ }^{-}\), forms ionic compounds with many metal ions. Assume that \(9.7416 \mathrm{~g} \mathrm{M}\left(\mathrm{CHO}_{2}ight)_{2}\) (where \(\mathrm{M}\)...
-
What are the 10 commandments of computer/information systems?
-
What are the differences between the Common Law and the Civil Law families? Do you agree with the claim that these laws are secular law ?
-
How can organizations effectively navigate the complexities of change management amidst turbulent market conditions and disruptive technological advancements?
-
What role does strategic communication play in facilitating successful change management, particularly in disseminating information, managing stakeholder expectations, and garnering buy-in from key...
-
Can you find a conflict/discrepancy between the Oregon Board of Marriage and Family Therapy laws for MFT's and the AAMFT Code of Ethics, in regards to confidentiality?
-
How can data analytics and predictive modeling be integrated into change management processes to anticipate potential roadblocks, assess the impact of change initiatives, and optimize decision-making?
-
At a total cost of $660,000, Penn Corporation acquired 60,000 shares of Teller Corp. common stock as a long-term investment. Penn Corporation uses the equity method of accounting for this investment....
-
This problem continues the Draper Consulting, Inc., situation from Problem 12-45 of Chapter 12. In October, Draper has the following transactions related to its common shares: Oct 1 Draper...
-
Repeat Example 15.24, except for a feed containing 400 ppm (by weight) of CaCl2 and 50 ppm of NaCl. Example 15.24 Hard water containing 500 ppm (by weight) MgCO 3 and 50 ppm NaCl is to be softened at...
-
In Examples 15.13 and 15.18, benzene is adsorbed from air at 70 F in a 6-ft-high bed of silica gel and then stripped with air at 145F. If the bed height is changed to 30 ft, the following data are...
-
A 55 mol% propane and 45 mol% propylene gas mixture is to be separated into products containing 10 and 90 mol% propane by adsorption in a continuous, countercurrent system operating at 25C and 1 atm....
-
An organization may be following the necessary steps to ensure security, to include building security and risk management programs, training staff, and performing data protection. How can management...
-
A paper company needs to ship paper to a large printing business. The paper will be shipped in small boxes and large boxes. The volume of each small box is 6 cubic feet and the volume of each large...
-
Tax carrybacks are a strategic financial tool used by businesses to optimize their tax liabilities. This accounting method allows companies to apply current year losses to previous years' taxable...

Study smarter with the SolutionInn App


