Methanol is a high-octane fuel used in high-performance racing engines. Calculate G for the reaction given the
Question:
Methanol is a high-octane fuel used in high-performance racing engines. Calculate ΔG° for the reaction
![]()
given the following free energies of formation:
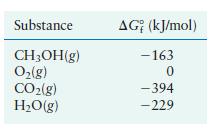
Transcribed Image Text:
2CHOH(g) + 30(g) 2CO(g) + 4HO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
We use AG EAG products AG reactants 2AG COg 4AG H...View the full answer

Answered By

Muhammad Rehan
Enjoy testing and can find bugs easily and help improve the product quality.
4.70+
10+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
The following data compare the standard enthalpies and free energies of formation of some crystalline ionic substances and aqueous solutions of the substances: (a) Write the formation reaction for...
-
In the Mond process for the purifi cation of nickel, carbon monoxide is reacted with heated nickel to produce Ni(CO)4, which is a gas and can therefore be separated from solid impurities: Ni(s) +...
-
Changing preferences can also affect changes in land use. In the United States, the proportion of the population in the 65-and-older age bracket is growing. What effects might this have on the...
-
Consider a projectile fired vertically in a constant gravitational field. For the same initial velocities, compare the times required for the projectile to reach its maximum height (a) For zero...
-
a. Determine the gage pressure at point A in Fig. 3.36. b. If the barometric pressure is 737 mm of mercury, express the pressure at point A in kPa(abs). Water 215 mm Mercury (sg = 13.54) 600 mm
-
Juliette Shulof Furs (JSF) was a New York corporation that had been in the fur-dealing business for 15 years. George Shulof, an officer of JSF, attended two auctions conducted by Finnish Fur Sales...
-
Assume that the nation of Spain is small and unable to influence the Brazilian (world) price of steel. Spains supply and demand schedules are illustrated in Table. Assume that Brazils price is $400...
-
First Draw an ER Model please and then covert it into relational model seperate figures required thank you Each cinema is identified by its name and has its residency at an address which consists of...
-
A chemical engineer wants to determine the feasibility of making ethanol (C 2 H 5 OH) by reacting water with ethylene (C 2 H 4 ) according to the equation Is this reaction spontaneous under standard...
-
Using the following data (at 25C),
-
A proton with speed v = 3.00 x 105 m/s orbits just outside a charged sphere of radius r = 1.00 cm. What is the charge on the sphere?
-
What would be the Viability if we export wood from canada to india?
-
Develop and elaborate a financial plan with short-term financial goals and a plan for a fresh graduate. You must identify and discuss the career choice of the fresh graduate. The plan must include...
-
When Ricardo was 9 years old, he was 56 inches tall. Ricardo is now 12 years old and he is 62 inches tall. Find the percent of increase in Ricardo's height to the nearest tenth
-
Assume that you plan to save $15,000 per year for the next 25 years. Using this financial resource, you wish to fund your retirement expenditure for exactly 20 years; and, assume that the annual...
-
What should you do if you have multiple personal commitments as well as study commitments?
-
Tiger Furnishings produces two models of cabinets for home theater components, the Basic and the Dominator. Data on operations and costs for March follow: Required Compute the predetermined overhead...
-
SBS Company have received a contract to supply its product to a Health Care Service Hospital. The sales involve supplying 1,250 units every quarter, the sales price is RM 85 per unit. The Client...
-
What is the molality of acetone, C 3 H 6 O, in an aqueous solution for which the mole fraction of acetone is 0.112?
-
Calculate the standard Gibbs free energy for each of the following reactions: 2 HI(g), K = 54 at 700. K (a) H(g) + 1(g) (b) CCl3COOH (aq) + HO(1) = CClCO (aq) + H3O+ (aq), K = 0.30 at 298 K
-
Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron, and toxic and malodorous chemicals, such as H 2 S. If you are using permanganate...
-
Property in the hands of a taxpayer not engaged in real estate business is treated as a CAPITAL ASSET for tax purposes. Select one: True FalseThe capital asset is defined under our Philippine Tax...
-
Sally has been looking at properties for the restaurant with a realtor. She asks whether you believe the company's financial position would support purchasing or leasing a property. You both have...
-
Acme, Inc. instructed its bank, AJM Bank, to pay $500,000 to TreeTop, Inc. TreeTop, Inc. was a customer of First State Bank. AJM Bank executed Acme, Inc.'s payment order by issuing its own payment...

Study smarter with the SolutionInn App


