The phase diagram for carbon, shown here, indicates the extreme conditions that are needed to form diamonds
Question:
The phase diagram for carbon, shown here, indicates the extreme conditions that are needed to form diamonds from graphite.
(a) At 2000 K, what is the minimum pressure needed before graphite changes into diamond?
(b) What is the minimum temperature at which liquid carbon can exist at pressures below 10 000 atm?
(c) What is the temperature and pressure of the diamond, liquid, and graphite triple point?
(d) Are diamonds stable under normal conditions? If not, why is it that people can wear them without having to keep them under high pressure?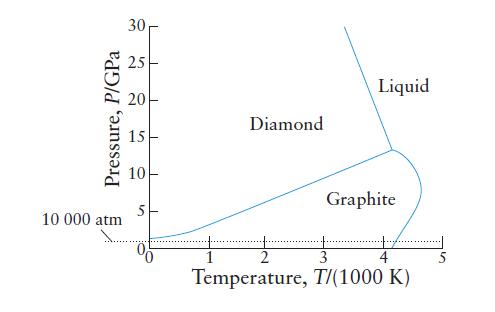
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





