The decomposition of N 2 O 5 in the gas phase was studied at constant temperature: The
Question:
The decomposition of N2O5 in the gas phase was studied at constant temperature:
![]()
The following results were collected:
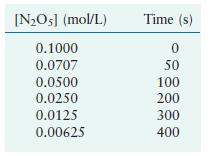
Using these data, verify that the rate law is first order in [N2O5], and calculate the value of the rate constant, where the rate = –d[N2O5]/dt.
Transcribed Image Text:
2NOs(g) 4NO(g) + O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
We can verify that the rate law is first order in NO5 by ...View the full answer

Answered By

Muhammad Salman Alvi
Well, I am a student of Electrical Engineeing from Information Technology University of Punjab. Just getting into my final year. I have always been good at doing Mathematics, Physics, hardware and technical subjects. Teaching profession requires a alot of responsibilities and challenges.
My teaching experience started as an home tutor a year ago. When I started teaching mathematics and physic subjects to an O Level student. He was about 14 years old. His name was Ibrahim and I used to teach him for about 2 hours daily. Teaching him required a lot of patience but I had to be polite with him. I used to give him a 5 min break after 1 hour session. He was quite weak in basic maths and calculation. He used to do quite a lot of mistakes in his homework which I gave him weekly. So I decided to teach him basics from scratch. He used to say that he got the concept even if he didn't. So I had to ask him again and again. I worked on his basics for a month and after that I started taking a weekly test sesions. After few months he started to improve gradually. Now after teaching him for about a year I can proudly say that he has improved alot. The most important thing was he managed to communicate all the difficullties he was facing. He was quite capable and patient. I had a sincere desire to help him reach to its full potential. So I managed to do that. We had a very good honest relationship of a student and a teacher. I loved teaching him as a tutor. Now having an experience of one year teaching I can read students quite well. I look forward to work as an online tutor who could help students in solving their all sort of difficulties, problems and queries.
4.90+
29+ Reviews
43+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the data given in Example 15.2, calculate [N 2 O 5 ] 150. s after the start of the reaction. Data from Example 15.2 The decomposition of N 2 O 5 in the gas phase was studied at constant...
-
The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 -- 4 NO2 + O2. The rate law is first order in N2O5. At the rate constant is 4.82 10-3 s-1. (a) Write the rate law for the...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Use the DerivaGem software to value a five-year collar that guarantees that the maximum and minimum interest rates on a LIBOR-based loan (with quarterly resets) are 7% and 5% respectively. The LIBOR...
-
(a) Solve the differential equation y = 2x1 y2. (b) Solve the initial-value problem y = 2x1 y2, y (0) = 0, and graph the solution. (c) Does the initial-value problem y = 2x1 y2, y (0) = 2, have a...
-
For what numbers x, -2 x 2, does the graph of y = csc x have vertical asymptotes? If necessary, refer to the graphs to answer each question.
-
Compare and contrast the ethical approaches of its legal, therefore, its ok and the ends justify the means. Are there similarities? Are there differences?
-
Donnie Hilfiger has two classes of stock authorized: $1 par preferred and $0.01 par value common. As of the beginning of 2015, 300 shares of preferred stock and 4,000 shares of common stock have been...
-
Yvonne can use two coupons for the same purchase at her favorite department store. One coupon gives her $20 off and the other gives her 25% off. She wants to buy a bedspread that sells for...
-
A certain first-order reaction has a half-life of 20.0 minutes. a. Calculate the rate constant for this reaction. b. How much time is required for this reaction to be 75% complete?
-
The reaction between bromate ions and bromide ions in acidic aqueous solution is given by the following equation: Table 15.5 gives the results of four experiments involving this reaction. Using these...
-
Geometrically, how do joints propagate?
-
The automobile industry in the next 20 years will look very different from how it has looked over the last 100 years. Since the establishment of the automobiles dominant design in the 1920s, the...
-
Twitter (http://twitter.com) is a free networking and micro-blogging service that allows its users to send and read other users updates (otherwise known as tweets). Although Twitter has millions of...
-
Assume that you just invented a new type of computer printer that can be easily folded up and carried like a laptop computer. You have decided to start a company to produce the printer. Select a name...
-
Spend some time looking at Red Bulls Web site (the U.S. site). Comment on each element of Red Bulls marketing mix (product, price, promotion, and place in terms of distribution and sales). If you...
-
Look at the Web site of Scuba Toys (www.scubatoys.com). As youll see, this firm makes a wide range of products for all types of water sports. Spend some time familiarizing yourself with Scuba Toys...
-
Astle Manufacturing Company manufactures and leases a variety of items. On January 2, 2008, Astle leased a piece of equipment to Haws Industries Co. The lease is for six years for an annual amount of...
-
Using the theoretical sampling strategy, how many samples of size 4 (n = 4) can be drawn from a population of size: (a) N = 5? (b) N = 8? (c) N = 16? (d) N = 50?
-
Draw the Lewis structure and predict the shape of (a) OSbCl 3 ; (b) SO 2 Cl 2 ; (c) IO2F 2 . The atom in boldface red type is the central atom.
-
Structural isomers are molecules that have the same composition but a different pattern of connectivity. Two isomers of disulfur difluoride, S 2 F 2 , are known. In each, the two S atoms are bonded...
-
Determine the formal charge on each atom in the following molecules. Identify the structure of lower energy in each pair. (a) =-: || | :0: H (b) =c=S (c) H-C=N: :0a: T :0: :8-c=s: | H H-CIN
-
The following information provides the amount of cost incurred in March for the cost items indicated. During March, 8,100 units of the firm's single product were manufactured. Raw materials Factory...
-
Part 1: Use the information in Part 1 for GEM Company to complete a Cost of Goods Manufactured Statement and a Cost of Goods Sold Statement for the year ended 2022. All reports should be prepared in...
-
Slim Jim, continually maintaining his svelte body, lifts a 7 0 kg barbell 1 . 4 m above the ground.A . How much energy did the barbell have when it was on the ground at rest?B . What kind of energy...

Study smarter with the SolutionInn App


