Question: Using data from Table 5G.2 and standard graphing software, determine the standard enthalpy and entropy of the reaction N 2 O 4 (g) 2
Using data from Table 5G.2 and standard graphing software, determine the standard enthalpy and entropy of the reaction N2O4(g) → 2 NO2(g) and estimate the N—N bond enthalpy in N2O4. How does this value compare with the mean N—N bond enthalpy in Table 4E.3?
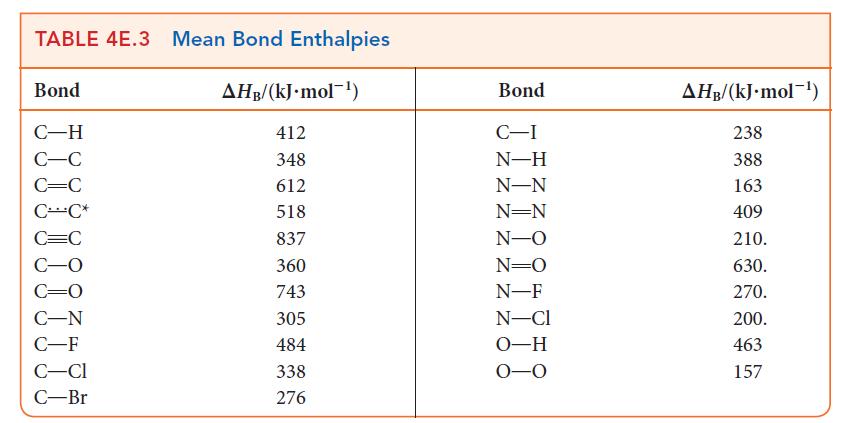
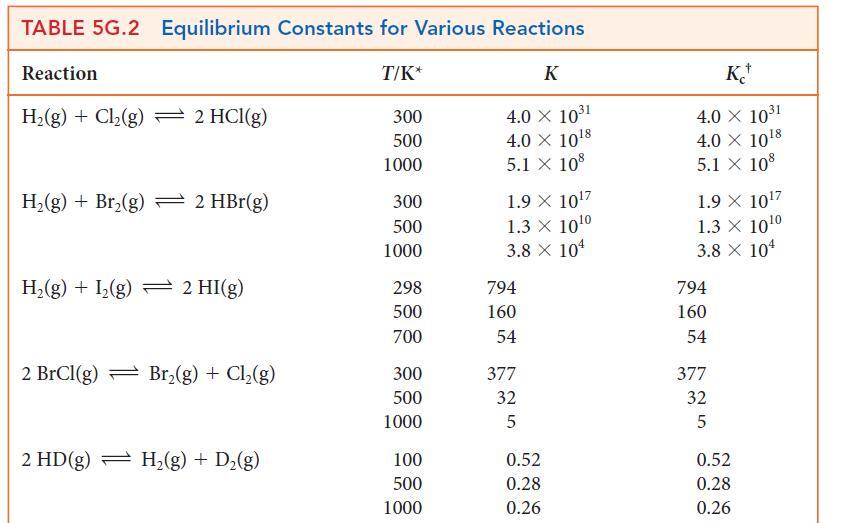
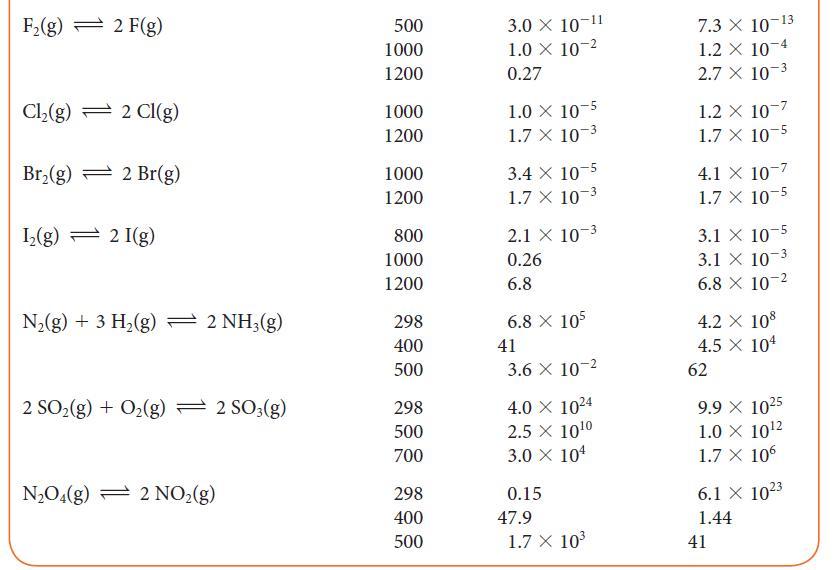
TABLE 4E.3 Mean Bond Enthalpies AHB/(kJ. mol) Bond C-H C-C C=C CC* C-F C-CI C-Br 412 348 612 518 837 360 743 305 484 338 276 Bond C-I N-H N-N N=N N-O N=O N-F N-Cl O-H 0-0 AHB/(kJ.mol-) 238 388 163 409 210. 630. 270. 200. 463 157
Step by Step Solution
3.30 Rating (150 Votes )
There are 3 Steps involved in it
The graph is generated for In K vs 1T according to this ... View full answer

Get step-by-step solutions from verified subject matter experts


