Estimate the UFL and the LFL for ethylene using the stoichiometric concentrations and Equations 6-10 and 6-11
Question:
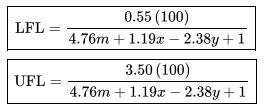
Estimate the UFL and the LFL for ethylene using the stoichiometric concentrations and Equations 6-10 and 6-11 in the textbook. Compare to the experimental values in Appendix B.
Data From Appendix B:
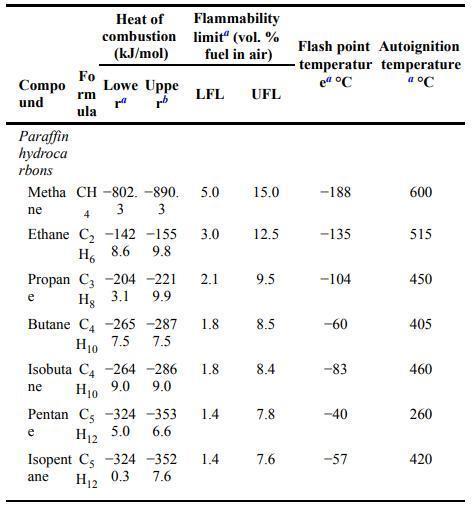
Equation 6-10 and 11:
Transcribed Image Text:
Paraffin hydroca rbons Fo rm ula Compo Lowe Uppe und Heat of combustion (kJ/mol) Flammability limit" (vol. % fuel in air) Metha CH-802. -890. 5.0 ne 3 3 e LFL 4 Ethane C -142 -155 3.0 H 8.6 9.8 Pentan C -324 -353 6.6 H12 5.0 Propan C3 204 -221 2.1 9.9 Hg 3.1 e Butane C4 -265-287 1.8 7.5 H10 7.5 Isobuta C4 -264 -286 1.8 8.4 ne H0 9.0 9.0 1.4 UFL Isopent C -324 -352 1.4 ane H12 0.3 7.6 15.0 12.5 9.5 8.5 7.8 7.6 Flash point Autoignition temperatur temperature el C a C -188 -135 -104 -60 -83 -40 -57 600 515 450 405 460 260 420
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To calculate the Lower Flammability Limit LFL and Upper Flammability Limit UFL for ethylene using the given equations we need to know the stoichiometr...View the full answer

Answered By

Joseph Ogoma
I have been working as a tutor for the last five years. I always help students to learn and understand concepts that appears challenging to them. I am always available 24/7 and I am a flexible person with the ability to handle a wide range of subjects.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:
Students also viewed these Engineering questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Estimate the upper and lower flammable limits for carbon monoxide and heptane using the stoichiometric method via Equations 6-10 and 6-11 in the text. Compare to experimental values provided in...
-
Molecular simulation can be used to explore the accuracy and significance of individual contributions to an equation of state. Use the DMD module at Etomica.org to explore Xes energy departure. (a)...
-
A certain apple bruises if a net force greater than 9 . 5 N is exerted on it . Would a 0 . 1 3 k g apple be likely to bruise if it falls 1 . 8 m and stops after sinking 0 . 0 5 m into the grass?...
-
Determine the volume of the solid generated by rotating the semi elliptical area shown about (a) The axis AA², (b) The axis BB², (c) The y axis.
-
A microbrewery was built in 2012 at a total cost of $650,000. Additional information is given in the accompanying table (all 2000 indices = 100). a. Calculate a weighted index for microbrewery...
-
North Shore Architectural Stone, Inc., a company that installs limestone in residential and commercial buildings, agreed to supply and install limestone for a property owned by Joseph Vitacco. North...
-
Pedriani Manufacturing uses a job order cost system and applies overhead to production on the basis of direct labor hours. On January 1, 2012, Job No. 25 was the only job in process. The costs...
-
$400,000 for a new technology.The lender charges them 36% annually with monthly compounding.The agreement calls for no payment until the end of the first month of the 5th year with equal monthly...
-
Estimate the LOC of ethylene using Equations 6-15 and 6-16 in the textbook. Compare to the experimental value in Table 6-3. Table 6-3: Equation 6-15: Equation 6-16: Gas or vapor Methane Ethane...
-
A gas cylinder contains a gas mixture composed of \(50 \%\) methane and \(50 \%\) ethylene by volume. Estimate the LFL and the UFL for this gas mixture. Compare to the experimental values of \(3.6...
-
Within a relevant range, the amount of variable costs per unit. (a). Differs at each production level. (b). Remains constant at each production level (c). Increases as production increases. (d)....
-
Write an evaluation essay about a restaurant. Clearly defined criteria. For example, if you're evaluating a restaurant, what are you looking for? Ample parking? Good service? If it's a Mexican...
-
16. If the following statement were used in a C++ program, what would it cause to be written on the screen? cout < < "C++ is easy to understand.";
-
Can you help me find that information for Subway (the food company) ? The Insight stage, builds from introspection from the leader -" what is" then the individual team members finish by how they...
-
2. An automobile that has a 2-liter Inline 4-cylinder SI engine operates on a four-stroke cycle and is being tested at 2800 rpm. The compression ratio of the engine is = 8.5, the length of the...
-
In this podcast Suze was talking about the language of Money in retirement she talked alot about the different types of retirement in a private or public sector settings and how it might range...
-
At the end of the current year, Accounts Receivable has a balance of $325,000; Allowance for Doubtful Accounts has a credit balance of $3,900; and net sales for the year total $4,500,000. Using the...
-
Citing a scientific article, explain in your own words, how DNA fingerprinting has been used in forensic science to solve crimes and why it may not always be accurate or effective.
-
Referring to the description in Problem P3.16, if the viscosity of water is 0.01 poise, determine the value in terms of the units (a) Slug/(ft s) (b) kg/(m s). Problem P3.16 The property of a fluid...
-
The fuel efficiency of an aircrafts jet engines is described by the thrust-specific fuel consumption (TSFC). The TSFC measures the rate of fuel consumption (mass of fuel burned per unit time)...
-
An automobile engine is advertised as producing a peak power of 118 hp (at an engine speed of 4000 rpm) and a peak torque of 186 ft lb (at 2500 rpm). Express those performance ratings in the SI...
-
What is a quantum computer? What distinguishes a quantum computer from a digital one?
-
Fred created a unique line of shoes. He wanted to give them a name that everyone would recognize, so he branded them Shoes! Would Fred be able to successfully register his trademark? Why or Why not?
-
The frequency table below shows the number of hours spent learning a new song. # of hours Midpoint O up to 3 3 up to 6 Frequency 3 6 up to 9 9 up to 12 6 15 10 12 up to 15 7 15 up to 18 2 a) Show how...

Study smarter with the SolutionInn App


