Question: Repeat the previous problem for a mixed flow reactor. The data in Table P5.28 have been obtained on the decomposition of gaseous reactant A in
Repeat the previous problem for a mixed flow reactor.
The data in Table P5.28 have been obtained on the decomposition of gaseous reactant A in a constant volume batch reactor at 100°C. 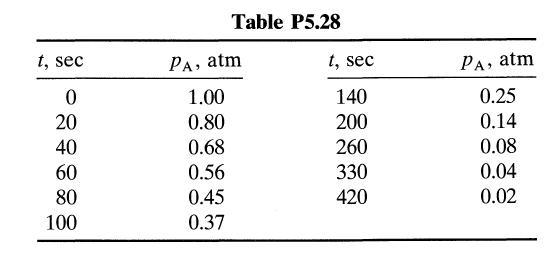
The stoichiometry of the reaction is 2A → R + S. What size plug flow reactor (in liters) operating at 100°C and 1 atm can treat 100 mol A/hr in a feed consisting of 20% inerts to obtain 95% conversion of A?
t, sec 0 20 40 60 80 100 Table P5.28 PA, atm 1.00 0.80 0.68 0.56 0.45 0.37 t, sec 140 200 260 330 420 PA, atm 0.25 0.14 0.08 0.04 0.02
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
To determine the size of a plug flow reactor PFR needed to achieve 95 conversion of reactant A we need to follow these steps Step 1 Calculate the Rate ... View full answer

Get step-by-step solutions from verified subject matter experts


