The exothermic reaction of stillbene (A) to form the economically important trospophene (B) and methane(C), that is,
Question:
The exothermic reaction of stillbene (A) to form the economically important trospophene (B) and methane(C), that is, A → B+ C was carried out adiabatically and the following data recorded: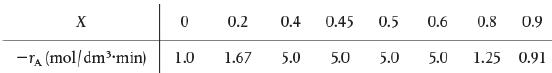
The entering molar flow rate of A was 300 mol/min.
1. Calculate the batch reactor (BR) times to achieve 40% and 80% conversion when 400 moles of A are charged to a 400 dm3 reactor.
2. What are the PFR and CSTR volumes necessary to achieve 40% conversion?
3. Over what range of conversions would the CSTR and PFR reactor volumes be identical?
4. What is the maximum conversion that can be achieved in a 105-dm3 CSTR?
5. What conversion can be achieved if a 72-dm3 PFR is followed in series by a 24-dm3 CSTR?
6. What conversion can be achieved if a 24-dm3 CSTR is followed in a series by a 72-dm3 PFR?
7. Plot the conversion and rate of reaction as a function of PFR reactor volume up to a volume of 100 dm3.
Step by Step Answer:






