An electrochemical cell is based on these two half-reactions: Calculate the cell potential at 25 C. 2+
Question:
An electrochemical cell is based on these two half-reactions: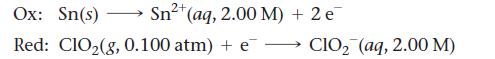
Calculate the cell potential at 25 °C.
Transcribed Image Text:
2+ Sn²+ (aq, 2.00 M) + 2 e Ox: Sn(s) Red: ClO₂(g, 0.100 atm) + e- CIO₂ (aq, 2.00 M)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Sure to calculate the cell potential Ecell for the electrochemical cell you can use the ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An electrochemical cell is based on these two half-reactions: Calculate the cell potential at 25 C. Ox: Pb(s) Pb+ (aq, 0.10 M) + 2 e Red: MnO4 (aq, 1.50 M) + 4H* (aq, 2.0 M) + 3 e MnO (s) + 2 HO(1)
-
An electrochemical cell is constructed such that on one side a pure nickel electrode is in contact with a solution containing Ni2+ ions at a concentration of 3 10-3 M. The other cell half consists...
-
The reaction taking place in an electrochemical cell under standard conditions is Fe 2+ (aq) + Ag + (aq) Fe 3+ (aq) + Ag(s) a. Write two half-equations for this reaction. For each, state whether...
-
A UK company has a trading loss of 50,000 for the year to 31 March 2021. During the year, the company receives overseas property income (net of 40% withholding tax) of 12,000. Show the corporation...
-
1. A unit-level driver is consumed by a product each and every time that a. a batch of products is produced. b. a purchase order is issued. c. a unit is produced. d. a customer complains. e. none of...
-
The accounting records of Hampton Company provided the data below ($ in 000s). Required: Prepare a reconciliation of net income to net cash flows from operatingactivities. Net income Depreciation...
-
Find an article about writing summary judgment motions from the courts perspective. What tips are given for writing a summary judgment motion?
-
In the project environment, cause-and-effect relationships are almost always readily apparent. Good project management will examine the effect in order to better understand the cause and possibly...
-
explain the penalties that are given if they fail to achieve the targets in a ladies medical center?
-
What is a concentration electrochemical cell?
-
Explain the difference between a voltaic (or galvanic) electrochemical cell and an electrolytic cell.
-
(a) Because an exact outer boundary cannot be measured or even calculated for an atom, how are atomic radii determined? (b) What is the difference between a bonding radius and a nonbonding radius?...
-
Although this talk is mostly about the personal finance crisis impacting older generations and Baby Boomers, what about the personal finance crisis impacting younger people like yourself? Why do you...
-
Laine Ltd. is a Canadian-controlled private corporation. Its non-eligible refundable dividend tax on hand (NERDTOH) account at December 31, 2021 was $35,000. For its 2022 taxation year, its...
-
E. (Simple Interest) A bank is offering 3.5% simple interest on a savings account. If you deposit Php7,500, how much interest will you earn in two years? 1 = prt
-
Since the Drug-Free Workplace Act of 1988 many U.S. employers have chosen to conduct drug testing as a pre-employment requirement, random testing of current employees, or a required test after a...
-
A gear pump has a 80 mm outside diameter, a 55 mm diameter, and a 25 mm width. If the actual pump flow rate at 2300 rpm and rated pressure is 140 LPM, calculate the Volumetric efficiency.
-
Which entities must follow ASPE and IFRS? Why would an entity that isnt required to follow ASPE or IFRS do so? If an entity doesnt follow ASPE or IFRS, how would it prepare its financial statements?
-
You are the newly appointed tax practitioner to complete Emilys tax return and have downloaded the prefill report for Emilys tax return (hint, you can read what a prefill report is here (Links to an...
-
Describe what happens to the vapor pressure of water as the temperature increases.
-
Describe why it is important to consider NPSH when designing and operating a pumping system.
-
Find the available NPSH when a pump draws water at 140F from a tank whose level is 4.8 ft below the pump inlet. The suction line losses are 2.2 lb-ft/lb and the atmospheric pressure is 14.7 psia.
-
1.The nurse is caring for a patient following painful radiation treatment for newly diagnosed cancer. Which question, if asked by the nurse in the orientation phase of the nursepatient relationship,...
-
Discuss the positive and negative job characteristics, as found in the Job Characteristics Model (McShane, 2021, p.155-156), of Sophia's secondment (Difficult Connection Mcshane).
-
1.What are the problems with variables? (Select all that apply.) a.Vary from subject to subject b.Determined through statistics c.Difficult to account for them d.Challenging to explain in relation to...

Study smarter with the SolutionInn App


