Calculate the change in entropy that occurs in the system when 1.00 mole of isopropyl alcohol (C
Question:
Calculate the change in entropy that occurs in the system when 1.00 mole of isopropyl alcohol (C3H8O) melts at its melting point (-89.5 °C). See Table 12.9 for heats of fusion.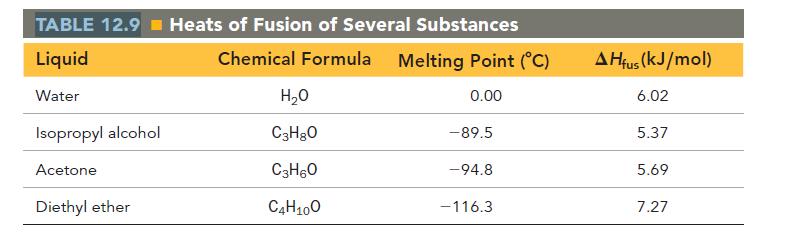
Transcribed Image Text:
TABLE 12.9 Heats of Fusion of Several Substances Liquid Chemical Formula Melting Point (°C) Water 0.00 Isopropyl alcohol Acetone Diethyl ether H₂O C3H8O C3H6O C4H100 -89.5 -94.8 -116.3 AHfus (kJ/mol) 6.02 5.37 5.69 7.27
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in entropy that occurs in the system when 45.0 g of acetone (C 3 H 6 O) freezes at its melting point (-94.8 C). See Table 12.9 for heats of fusion. TABLE 12.9 Heats of Fusion of...
-
Calculate the change in entropy that occurs in the system when 1.00 mole of diethyl ether (C 4 H 10 O) condenses from a gas to a liquid at its normal boiling point (34.6 C). See Table 12.7 for heats...
-
Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from a liquid to a gas at its boiling point (100.0 C). See Table 12.7 for heats of vaporization. TABLE 12.7...
-
Question 2: Consider the market for Florida oranges. The demand for Florida oranges is given by the inverse demand function p = 70-2Q The market cost function for firms that sell Florida oranges is...
-
Discuss the problems of gathering secondary data in foreign markets.
-
True or False: 1. Rationality could not apply to criminals. 2. Economic theories do not abstract from the particular details of situations so they can better focus on every aspect of the behavior to...
-
Lockridge-Priest, Inc., was organized in 2008. At December 31, 2008, the Lockridge-Priest balance sheet reported the following stockholders' equity: Requirements 1. During 2009 , the company...
-
Resolve Problem 5-29 with = 0.3. Using MAD, which smoothing constant provides a better forecast?
-
Scenario 1 - Analytics in Decision Making.Ms. Cook is planning to start a meal delivery service in Boston. Being very thorough with her planning, she conducted a survey of 1000 potential customers...
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. Mg(s) + Cl(g) MgCl(s) b. 2 HS(g) + 3 O(g) 2 HO(g) + 2 SO(g) c. 203(g) d. HCI(g) + NH3(g) NH4Cl(s) 3 O(g)
-
Two systems, each composed of three particles represented by circles, have 30 J of total energy. In how many energetically equivalent ways can you distribute the particles in each system? Which...
-
Kiran Shah, the new controller of Ginarrbrik Company, has reviewed the expected useful lives and salvage values of selected depreciable assets at the beginning of 2012. His findings are as follows....
-
It was Thursday night, but Adam was not in the headspace to celebrate. He had just heard the news that his colleague, Sami, received the big promotion that he had been working very hard for since his...
-
A 7500-kg rocket blasts off vertically from the launch pad with a constant upward acceleration of 4.00 m/sand feels no appreciable air resistance. When it has reached a height of 525 m, its engines...
-
On the first day of Year Two, the Richmond Corporation holds accounts receivable of$400,000 and an allowance for doubtful accounts of $23,000 for a net realizable value of$377,000. During the year,...
-
Explain the general functions and authority of the EEOC. Provide a brief overview of your chosen EEOC case. ( my case below) What is case name? What were the major facts of the case? What was the...
-
A contractor entered into a contract in the FIDIC form for the construction of a new bridge. In the course of the work the new bridge was to cross over a line of an old sewer which lies under a road...
-
Selected accounts from the chart of accounts of Mercer Company are shown below. 101 Cash 112 Accounts Receivable 120 Inventory 126 Supplies 157 Equipment 201 Accounts Payable 401 Sales Revenue 412...
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
Two monochromatic point sources radiate in-phase. At the usual distant plane of observation (parallel to the line connecting the sources) the irradiance from one of them is 100 times the irradiance...
-
With Fig. 12.3 in mind, establish that when two incoherent cosine-squared fringe systems, each of the form I 0 cos 2 α, overlap so that peaks fall on troughs, the resultant is I = I 0 -...
-
Show that Eq. (12.2) is reasonable. Then approximating A s as d 2 s , show that Notice that A c gets larger as l gets larger. Ac = (Ao/0,)? (12.2) Ac
-
4) The following table gives the maximum recommended exposure time to a noise level in dB. Write a method that takes the decibel level and returns the maximum exposure time. Use nested conditionals...
-
Discuss the implications of Quality by Design (QbD) in bioprocessing for regulatory compliance and process robustness. How do risk assessment and design of experiments (DoE) play a role in ensuring...
-
The inventory records of Kuffel Company reflected the following information for the year ended December 31, 2022: Number of Unit Date Transaction Units Cost Total Cost 1/1 Beginning inventory 150 $...

Study smarter with the SolutionInn App


