Calculate the change in entropy that occurs in the system when 1.00 mole of diethyl ether (C
Question:
Calculate the change in entropy that occurs in the system when 1.00 mole of diethyl ether (C4H10O) condenses from a gas to a liquid at its normal boiling point (34.6 °C). See Table 12.7 for heats of vaporization.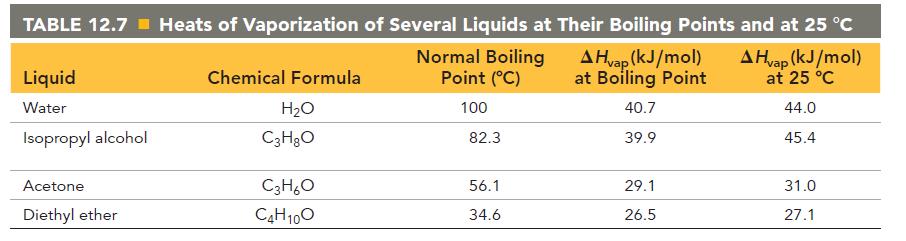
Transcribed Image Text:
TABLE 12.7 Heats of Vaporization of Several Liquids at Their Boiling Points and at 25 °C A Hvap (kJ/mol) at Boiling Point A Hvap (kJ/mol) at 25 °C 40.7 44.0 39.9 45.4 Liquid Water Isopropyl alcohol Acetone. Diethyl ether Chemical Formula H₂O C3H₂O C3H₂O C4H10O Normal Boiling Point (°C) 100 82.3 56.1 34.6 29.1 26.5 31.0 27.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
According to Table 127the heat of vaporization of diethyl ether at its normal b...View the full answer

Answered By

Sumit kumar
I am an experienced online essay writer with a thorough understanding of any curriculum.and subject expert at Chegg for mathematics, CS subjects..
4.90+
5+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from a liquid to a gas at its boiling point (100.0 C). See Table 12.7 for heats of vaporization. TABLE 12.7...
-
Calculate the change in entropy that occurs in the system when 45.0 g of acetone (C 3 H 6 O) freezes at its melting point (-94.8 C). See Table 12.9 for heats of fusion. TABLE 12.9 Heats of Fusion of...
-
Calculate the change in entropy that occurs in the system when 1.00 mole of isopropyl alcohol (C 3 H 8 O) melts at its melting point (-89.5 C). See Table 12.9 for heats of fusion. TABLE 12.9 Heats of...
-
Smile is a social media site that is growing in popularity. The firm has been around for a few years and has created a nice market niche for itself. Now the company has a possible buyer who has made...
-
In many cultures, personal information is inviolably private and absolutely not to be discussed with strangers. Discuss.
-
In the following table, task durations are given in weeks. The estimates were made at the 95 percent level (Calculating Probabilistic Activity Times subsection). a. Find the expected time and...
-
What stakeholder affected by their behavior was Gilead weighting very lightly when it decided what to tell the FDA about the medicines it wished to have permission to sell?
-
Christopher City received a contribution of $520,000 to provide scholarships to the children of deceased city employees. The donor stipulated that all income, including both realized and unrealized...
-
A 25-year-old Latin, G1P1, cisfemale presents to the office with a 6-month history of amenorrhea; reports having regular menstrual cycles every month up until about 1 year ago when her menstrual...
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. Mg(s) + Cl(g) MgCl(s) b. 2 HS(g) + 3 O(g) 2 HO(g) + 2 SO(g) c. 203(g) d. HCI(g) + NH3(g) NH4Cl(s) 3 O(g)
-
Two systems, each composed of three particles represented by circles, have 30 J of total energy. In how many energetically equivalent ways can you distribute the particles in each system? Which...
-
Each of the following independent events requires a year-end adjusting entry. Record each event and the related adjusting entry in general journal format. The first event is recorded as an example....
-
A brave new world of digitally enabled autonomous self thinking supply chains is emerging. We are excited by this - but there are also pitfalls that companies need to be aware of while embracing self...
-
Reflect upon the systems thinking methodologies and concepts introduced in the course, including SSM. How can these methodologies be applied to real-world problems? Give one example from each of the...
-
Some critics have claimed that Design Thinking is just another "flavor of the week" approach to solving problems. What evidence would you cite to disprove the critics?
-
Implementing the control process in everything you do. Thinking about what you learned this module reflect on the following: How could you use the concept of control in your personal life to increase...
-
Examine three schedule analysis techniques including the Critical Path, Critical Chain Method (CCPM), and Monte Carlo Analysis. Fully explaining and contrasting each of the techniques. Include the...
-
How are the following organizations and documents related? COSO The 1992 internal control framework The 2004 ERM integrated framework The 2013 updated framework The Securities and Exchange...
-
6 (a) Briefly develop a mathematical model of the behaviour of a copper-twisted pair cable (b) Derive the magnetic energy from: w given that: K + w, where the - - k symbols have their usual meaning...
-
Figure P.11.47 shows a function Æ(x) consisting of a periodic array of equally spaced delta functions. Construct its autocorrelation and discuss whether or not it is periodic. Figure P.11.47...
-
Imagine two uniformly illuminated small circular holes in an opaque screen, as shown in Fig. P.11.48. Construct its autocorrelation. Discuss the irradiance distribution for each resulting individual...
-
Consider the function in Fig. 11.49 as a cosine carrier multiplied by an exponential envelope. Use the frequency convolution theorem to evaluate its Fourier transform. Fig. 11.49
-
Edit question please, I need your take on this; needed some help. Thank you The opposite of me The Paper: Interview the person you chose about your differences with the goal of learning more about...
-
Assess the three organizations you researched in terms of their innovation to determine the impact the innovation has had on the organization. Provide specific examples to support your response....
-
Let (x) be a properly normalised wavefunction and an operator on wavefunctions. Let {q} be the spectrum of and {ur()} be the corresponding correctly normalised eigenfunctions. Write down an...

Study smarter with the SolutionInn App


